p-Block Elements Class 12 Notes
p-Block Elements Class 12 Notes
| The elements in which the last electron enters in the valence p-subshell are called the p-block elements. They include elements from groups 13 to 18. Their general electronic configuration is ns2np1-6 where n = 2 (except He which has 1s2 configuration). They, includes metals, non-metals and metalloids. |
Elements belonging to the s and p-blocks in the periodic table are called the representative elements or main group elements.
Inert pair effect: The tendency of ns2 electron pair to participate in a bond formation decreases with the increase in atomic size. Within a group, the higher oxidation state becomes less stable with respect to the lower oxidation state as the atomic number increases. This trend is called the ‘inert pair effect’. In other words, the energy required to unpair the electrons is more than the energy released in the formation of two additional bonds.
p-Block Elements Class 12 Notes
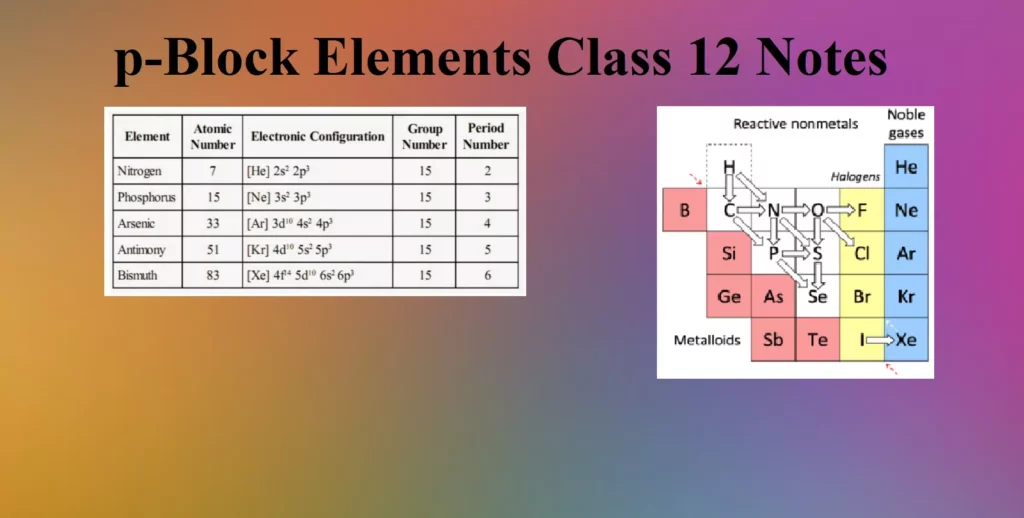
Group 15 Elements
The elements of group 15 – Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi). This group is also known as the Nitrogen family.
p-Block Elements Class 12 Notes
Nitrogen and phosphorus are non-metals, arsenic and antimony metalloids and bismuth is a typical metal. All the elements of this group are polyatomic. Dinitrogen is a diatomic gas while all others are solids.
The valence shell electronic configuration of these elements is ns2np3. The s orbitals in these elements is completely filled and p orbitals are half-filled, making their electronic configuration extra stable.
p-Block Elements Class 12 Notes
Nitrogen makes up about 0.002% of the earth’s crust; however, it constitutes 78% of the earth’s atmosphere by volume. Nitrogen is found naturally in animal and plant proteins and in fossilized remains of ancient plant life. Important nitrogen-containing minerals are niter, KNO3, and soda niter, NaNO3, which are found in desert regions and are important components of fertilizers.
Phosphorus is the eleventh most abundant element, making up 0.11% of the earth’s crust. The main source of phosphorus compounds is phosphorus rocks. Phosphorous is not found pure in nature, but in the form of apatite ores. These include compounds such as fluorapatite (Ca5(PO4)3F), which in fluoridated water is used to strengthen teeth, and hydroxylapatite (Ca10(OH)2(PO4)6), a major component of tooth enamel and bone material. Phosphorus has many applications: phosphorus trichloride (PCl3) is used in soaps, detergents, plastics, synthetic rubber nylon, motor oils, insecticides and herbicides; phosphoric acid, H3PO4, is used in fertilizers; phosphorus is also prevalent in the food industry, used in baking powders, instant cereals, cheese, the curing of ham, and in the tartness of soft drinks.
Arsenic is a highly poisonous metalloid. Because it is a metalloid, arsenic has a high density, moderate thermal conductivity, and a limited ability to conduct electricity. Compounds of arsenic are used in insecticides, weed killers, and alloys.
Antimony is obtained mainly from its sulfide ores, and it vaporizes at low temperatures. Along with arsenic, antimony is commonly used in alloys. Arsenic, antimony, and lead form an alloy with desirable properties for electrodes in lead-acid batteries. Arsenic and antimony are also used to produce semiconductor materials such as GaAs, GaSb, and InSb in electronic devices.
Bismuth is a poor metal (one with a significant covalent character) that is similar to both arsenic and antimony. Bismuth is commonly used in cosmetic products and medicine. Bismuth is obtained as a by-product of the refining of other metals, allowing other metals to recycle their by-products into bismuth.
p-Block Elements Class 12 Notes
(i) Atomic and Ionic Radii: Covalent and ionic (in a particular state) radius increase in size down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and/or f orbitals in heavier members.
(ii) Ionisation Enthalpy: It goes on decreasing down the group due to an increase in atomic size. Group 15 elements have higher ionization energy than group 14 elements due to the smaller size of group 15 elements. Group 15 elements have higher ionization energy than group 16 elements because they have stable electronic configuration i.e., half-filled p-orbitals. The order of successive ionization enthalpies, as expected is Δi H1 < Δi H2 < Δi H3.
(iii) Electronegativity: The electronegativity value, in general, decreases down the group with increasing atomic size. However, amongst the heavier elements, the difference is not that much pronounced.
Chemical Properties:
(i) Oxidation States and trends in a chemical reactivity: The common oxidation states of these elements are – 3, + 3, and + 5. The tendency to exhibit – 3 oxidation state decreases down the group, bismuth hardly forms any compound in –3 oxidation state. The stability of + 5 oxidation state decreases down the group. The only well-characterized Bi (V) compound is BiF5.
The stability of + 5 oxidation state decreases and that of +3 state increases (due to the inert pair effect) down the group.
Nitrogen exhibits +1, + 2, + 4 oxidation states also when it reacts with oxygen. Phosphorus also shows + 1 and + 4 oxidation states in some oxoacids.
In the case of nitrogen, all oxidation states from +1 to +4 tend to disproportionate in acid solution. For example 3 HNO2 → HNO3 + H2O + 2NO.
Nitrogen is restricted to a maximum covalency of 4 since only four (one s and three p) orbitals are available for bonding. The heavier elements have vacant d orbitals in the outermost shell which can be used for bonding (covalency) and hence, expand their covalence as in PF6–.
(ii) Anomalous Properties of Nitrogen: Nitrogen differs from the rest of the members of this group due to its smaller size, high electronegativity, high ionization enthalpy, and non-availability of d orbitals. Some of the anomalous properties shown by nitrogen are:
Nitrogen has the ability to form pπ–pπ multiple bonds with itself and with other elements like C and O. Other elements of this group do not form pπ–pπ bonds.
1. Nitrogen exists as a diatomic molecule with a triple bond (one s and two p) between the two atoms. So, its bond enthalpy is very high. While other elements of this group are polyatomic with single bonds.
2. The single N–N bond is weak. So, the catenation tendency is weaker in nitrogen.
3. Due to the absence of d orbitals in its valence shell, the maximum covalency of nitrogen is four. N cannot form dπ–pπ bond. While Phosphorus and arsenic can form dπ–dπ bond with transition metals and with C and O.
(iii) Reactivity Towards Hydrogen: All elements of group 15 react with Hydrogen to form Hydrides of the type EH3 (where E = N, P, As, Sb or Bi). They belong to sp3 hybridization. The stability of hydrides decreases down the group due to a decrease in bond dissociation energy down the group. NH3 > PH3 > AsH3 > SbH3 > BiH3.
iv) Bond angles: NH3 > PH3 > AsH3 > SbH3 > BiH3
Electronegativity of N is highest. Therefore, the lone pairs will be towards nitrogen and hence more repulsion between bond pairs. Therefore, the bond angle is the highest. After nitrogen, the electronegativity decreases down the group.
v) Bond Dissociation Enthalpy: NH3 > PH3 > AsH3 > SbH3 > BiH3
Bond dissociation enthalpy of E – H decreases from NH3 to BiH3 due to an increase in size and E – H bond length. So, the thermal stability also decreases from NH3 to BiH3.
vi) Boiling Point: PH3 < AsH3 < NH3 < SbH3 < BiH3
Boiling point increases with an increase in size due to an increase in the extent of van der Waal’s forces. The boiling point of NH3 is more because of hydrogen bonding.
vii) Basic Nature: Basic nature depends on the availability of lone pair of electrons. NH3 > PH3 > AsH3 > SbH3 > BiH3.
Size of the central atom increases and the availability of lp of e− for protonation decreases. So, the basic nature decreases.
viii) Reducing Nature: NH3 < PH3 < AsH3 < SbH3 < BiH3
Size of the central atom increases and the thermal stability decreases. Ease in the availability of hydrogen increases.
Ammonia forms hydrogen bonding with water molecules, therefore it is soluble in water, while other hydrides are insoluble in water.
p-Block Elements Class 12 Notes
Compounds and Properties of Nitrogen
Dinitrogen
Preparation of Dinitrogen: Dinitrogen is produced commercially by the liquefaction and fractional distillation of air. In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
NH4CI(aq) + NaNO2(aq) → N2(g) + 2H2O(l) + NaCl (aq)
It can also be obtained by the thermal decomposition of ammonium dichromate (NH4)2Cr2O7 →Heat N2 + 4H2O + Cr2O3
Very pure nitrogen can be obtained by the thermal decomposition of sodium or barium azide.
Ba(N3)2 → Ba + 3N2
Properties of Dinitrogen:
(i) Dinitrogen is inert at room temperature because of the high bond enthalpy of N≡N bond.
(ii) At higher temperatures, it directly combines with some metals to form ionic nitrides and with non-metals to form covalent nitrides.
(iii) N2 is a colourless, odourless gas insoluble in water.
(iv) It is neither combustible nor a supporter of combustion.
(v) 6Li + N2 →Heat 2Li3N; 3Mg + N2 →Heat Mg3N2
(vi) It combines with hydrogen at about 773 K in the presence of a catalyst Fe (Haber’s Process) to form ammonia: N2 +3H2 → 2NH3
(vii) Dinitrogen combines with dioxygen at very high temperature (at about 2000 K) to form nitric oxide N2 + O2 → 2 NO
Uses of Dinitrogen:
(i) The main use of dinitrogen is in the manufacture of ammonia and other industrial chemicals containing nitrogen (e.g., calcium cyanamide).
(ii) It also used to create an inert atmosphere in metallurgy.
(iii) Liquid dinitrogen is used as a refrigerant to preserve biological materials, food items and in cryosurgery.
p-Block Elements Class 12 Notes
Ammonia
Preparation: In the laboratory, ammonia is obtained by treating ammonium salts with caustic soda (NaOH) or slaked lime.
(NH4)2SO4 + 2NaOH → 2NH3 + 2H2O + Na2SO4
2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O + CaCl2
On a large scale, ammonia is manufactured by Haber’s process.
N2(g) + 3H2(g) → 2NH3(g)
In accordance with Le Chatelier’s principle, high pressure of about 200 atm, a temperature of about 773 K and a catalyst such as iron oxide with small amounts of K2O and Al2O3 are employed to increase the rate of this reaction.
Properties of Ammonia:
(i) Ammonia is a colourless gas with a pungent smell.
(ii) It is highly soluble in water because of its ability to form an intermolecular hydrogen bond with water.
(iii) Liquid ammonia has high melting and boiling points because of intermolecular hydrogen bonding.
(iv) The ammonia molecule has a trigonal pyramidal geometry. It has three bond pairs and one lone pair of electrons.
p-Block Elements Class 12 Notes
(v) Its aqueous solution is weakly basic due to the formation of OH– ions.
NH3(g) + H2O(l) → NH4+ (aq) + OH– (aq)
(vi) As a weak base, it precipitates the hydroxides of many metals from their salt solutions. For example,
2FeCl3 (aq ) + 3NH4OH (aq ) → Fe2O3.xH2O (s) + 3NH4Cl (aq)
ZnSO4 (aq) + 2NH4OH (aq) → Zn(OH)2 (s) + (NH4)2SO4 (aq)
(vii) The presence of a lone pair of electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base. It donates the electron pair and forms complex compounds with Cu2+, Ag+ etc. So, it is used for the detection of these metal ions.
Cu2+ (aq) + 4 NH3(aq) → [Cu(NH3)4]2+(aq)
(blue) (deep blue)
Ag+ ( aq) + Cl– (aq) → AgCl (s)
(colourless) (white ppt)
AgCl (s) + 2NH3 (aq ) → [Ag (NH3)2]Cl (aq)
(white ppt) (colourless)
Uses of Ammonia:
i) To produce various nitrogenous fertilizers(ammonium nitrate, urea, ammonium phosphate and ammonium sulphate).
ii) In the manufacture of nitric acid.
ii) liquid ammonia is used as a refrigerant.
iv) As a laboratory reagent.
v) In the production of artificial rayon, silk, nylon.
vi) For manufacture of HNO3by the Ostwald process.
p-Block Elements Class 12 Notes
Oxides of Nitrogen
Nitrogen forms a number of oxides in different oxidation states. They are:
(i) N2O → Laughing gas (Nitrous oxide): It is prepared by the action of any lighter metal with very dil. HNO3
4Sn(s) + 10HNO3 → 4Sn(NO3)2 + 5H2O + N2O
2NaNO2 + 2FeSO4 + 3H2SO4 → Fe2(SO4)3 + 2NaHSO4 + 2H2O + 2NO
2NO + N2O4 → 2 N2O3
2Pb(NO3)2 → 4 NO2 + 2 PbO + O2
It is an acidic brown gas. Its structure is:
4 HNO3 + P4O10 → 4 HPO3 + 2 N2O5
It is a colourless solid with acidic character. Its structure is:
p-Block Elements Class 12 Notes
Nitric Acid (HNO3)
Preparation: In the laboratory, nitric acid is prepared by heating KNO3 or NaNO3 and concentrated H2SO4 in a glass retort.
NaNO3 + H2SO4 → NaHSO4 + HNO3
On a large scale, it is prepared by Ostwald’s process. It involves the following steps:
Step 1: In this process, NH3 is catalytically oxidized to NO over a Pt-Rh catalyst at 1200K.
4NH3 + 5O2 → 4NO + 6H2O: DH = -904 kJ
Step 2: About 96 to 98 % of NH3 is converted into NO. The mixture is then diluted with air. NO combines with O2 to give NO2
2NO + O2 → 2NO2
Step 3: NO2 is absorbed in water to give HNO3 and NO, which is then recycled.
3NO2 + H2O → 2HNO3 + NO
Nitric acid can be concentrated to 68 % by distillation when a constant boiling mixture is formed. More concentrated acid can be made by distilling the mixture with concentrated sulphuric acid. Further concentration to 98% can be achieved by dehydration with concentrated H2SO4. 98% HNO3 is known as fuming nitric acid.
Properties of Nitric Acid:
(i) It is a colourless liquid. In the gaseous state.
(ii) HNO3 exists as a planar molecule with the structure as shown below:
(iii) In an aqueous solution, nitric acid behaves as a strong acid giving hydronium and nitrate ions.
HNO3(aq) + H2O(l) → H3O+(aq) + NO3– (aq)
(iv) Concentrated nitric acid is a strong oxidising agent and attacks most metals except noble metals such as gold and platinum.
(v) The products of oxidation depend upon the concentration of the acid, temperature and the nature of the material undergoing oxidation.
3Cu + 8 HNO3(dilute) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3(conc.) → Cu(NO3)2 + 2NO2 + 2H2O
(vi) Zinc reacts with dilute nitric acid to give N2O and with concentrated acid to give NO2.
4Zn + 10HNO3(dilute) → 4 Zn (NO3)2 + 5H2O + N2O
Zn + 4HNO3(conc.) → Zn (NO3)2 + 2H2O + 2NO2
(vii) Some metals (e.g., Cr, Al) do not dissolve in concentrated nitric acid because of the formation of a passive film of oxide on the surface.
(viii) Concentrated nitric acid also oxidises non–metals and their compounds. Iodine is oxidised to iodic acid, carbon to carbon dioxide, sulphur to H2SO4, and phosphorus to phosphoric acid.
I2 + 10HNO3 → 2HIO3 + 10 NO2 + 4H2O
C + 4HNO3 → CO2 + 2H2O + 4NO2
S8 + 48HNO3(conc.) → 8H2SO4 + 48NO2 + 16H2O
P4 + 20HNO3(conc.) → 4H3PO4 + 20 NO2 + 4H2O
Brown Ring Test: It is a test used for the detection of nitrates. The test is carried out by adding dilute ferrous sulphate solution to an aqueous solution containing nitrate ion and then carefully adding concentrated sulphuric acid along the sides of the test tube. A brown ring at the interface between the solution and sulphuric acid layers indicate the presence of nitrate ion in the solution.
NO3– + 3Fe2+ + 4H+ → NO + 3Fe3+ + 2H2O
[Fe (H2O)6 ]2+ + NO → [Fe (H2O)5 (NO)]2+ + H2O (Brown ring)
Uses of Nitric Acid:
i) In the manufacture of ammonium nitrate for fertilizers and other nitrates for use in explosives and pyrotechnics.
ii) For the preparation of nitroglycerin, trinitrotoluene and other organic nitro compounds.
iii) In the Pickling of Stainless Steel (chemical treatments applied to the surface of stainless steel to remove contaminants and assist the formation of a continuous chromium-oxide, passive film), etching of metals and as an oxidiser in rocket fuels.
p-Block Elements Class 12 Notes
Group 16 Elements and Properties of Oxygen
General Properties:
(i) Ionisation Enthalpy: Ionisation enthalpy of these elements decreases down the group. It is due to an increase in size. However, the elements of this group have lower ionisation enthalpy values compared to those of Group 15 elements. This is due to the fact that Group 15 elements have extra stable half-filled p orbitals electronic configuration.
The oxygen atom has less negative electron gain enthalpy than sulphur because of the compact nature of its shells due to which the electronic repulsion is greater.
(ii) Oxidation State: They show -2, +2, +4, +6 oxidation states. Oxygen does not show +6 oxidation state due to the absence of d – orbitals. Po does not show +6 oxidation state due to the inert pair effect.
The stability of -2 oxidation state decreases down the group due to an increase in atomic size and a decrease in electronegativity. Since the electronegativity of oxygen is very high, it shows only –2 oxidation state (except in the case of OF2 where its oxidation state is + 2).
+ 4 and + 6 are more common. Sulphur, selenium and tellurium usually show + 4 oxidation state in their compounds with oxygen and + 6 with fluorine. Down the group, the stability of + 6 oxidation state decreases and that of + 4 oxidation state increases (due to inert pair effect).
(iii) Electronegativity: It is the tendency of an atom to attract shared paired electrons towards itself. The order of electronegativity is O > S > Se > Te > Po (regular trend)
(iv) Electron gain enthalpy: Oxygen has less negative electron gain enthalpy than S because of the small size of O.
From S to Po electron gain enthalpy becomes less negative to Po because of an increase in atomic size. The order of Electron Gain Enthalpy is S > Se > Te > Po > O.
(v) Melting and boiling point: It increases with an increase in atomic number. Oxygen has much lower melting and boiling points than Sulphur because oxygen is diatomic (O2) and Sulphur is Octatomic (S8).
Chemical Properties:
(i) Hydrides: All the elements of group 16 from hydrides of the general formula H2E where E is the element belonging to group 16. Following are some of the characteristics of these hydrides: H2O, H2S, H2Se, H2Te.
- Their acidic character increases from H2O to H2Te. This is due to the decrease in bond (H–E) dissociation enthalpy down the group. H2Te > H2Se > H2S > H2O
- The thermal stability also decreases down the group due to an increase in bond length.
H2O > H2S > H2Se > H2Te
- All the hydrides except water possess the reducing property and this character varies in order. H2Te > H2Se > H2S.
- Bond angles H2O > H2S > H2Se > H2Te. This is due to repulsion between lone pairs.
(ii) Reactivity with Oxygen: All these elements form oxides of the EO2 and EO3 types where E = S, Se, Te or Po.
- There are two types of oxides – simple oxides(e.g., MgO, Al2O3) and mixed oxides (Pb3O4, Fe3O4).
- Simple oxides can be further classified on the basis of their acidic, basic or amphoteric character. An oxide that combines with water to give an acid is called acidic oxide. For example, Acidic oxides. CO2, SO2, SO3, N2O5, N2O3, P4O6, P4O10, Cl2O7, CrO3, Mn2O7, V2O5.
Cl2O7 + H2O → 2 HClO4
- Generally, non-metal oxides are acidic but oxides of some metals in higher oxidation states also have acidic character (e.g., Mn2O7, CrO3, V2O5).
Mn2O7 + H2O → 2 HMnO4
- The oxide which gives an alkali on dissolved in water is known as basic oxide(e.g., Na2O, CaO, BaO). Generally, metallic oxides are basic in nature. OR They either dissolve in water to form alkalies or combine with acids to form salts and water or combine with acidic oxides to form salts; e.g., Na2O, CaO. CuO, FeO, BaO etc.
Na2O + H2O → 2 NaOH
CaO + H2O → Ca(OH)2
CuO + H2SO4 → CuSO4 + H2O
- Besides MO2type oxides sulphur, selenium and tellurium also form MO3 type oxides (SO3, SeO3, TeO3). Both types of oxides are acidic in nature. Increasing order of acidic nature of oxides is TeO3 < SeO3 < SO3.
- Some metallic oxides exhibit a dual behaviour. They show the characteristics of both acidic and basic oxides. Such oxides are known as amphoteric oxides. They react with acids as well as to alkalis. E.g.: Al2O3, Ga2O3 OR These can combine with acids as well as bases for eg., ZnO, Al2O3, BeO, Sb2O3, Cr2O3, PbO etc.
PbO + 2 NaOH → Na2PbO2 + H2O
PbO + H2SO4 → PbSO4 + H2O
Cr2O3 + 2 NaOH → Na2Cr2O4 + H2O
Cr2O3 + 3 H2SO4 → Cr2(SO4)3 + 3 H2O
- There are some oxides that are neither acidic nor basic. Such oxides are known as neutral oxides. Examples of neutral oxides are CO, NO and N2
- Mixed Oxides: They behave as a mixture of two simple oxides, e.g., Pb3O4 (2PbO + PbO2), Fe3O4 (FeO + Fe2O3), Mn3O4 (2MnO + MnO2).
- SO2is a gas whereas SeO2 is solid. This is because SeO2 has a chain polymeric structure whereas SO2 forms discrete units.
- Reducing character of dioxides decreases down the group because oxygen has a strong positive field that attracts the hydroxyl group and removal of H+ becomes easy.
(iii) Reactivity Towards Halogens: Elements of group 16 form a larger number of halides of the type EX6, EX4 and EX2 where E is an element of the group and X is a halogen.
- The stability of halides decreases in the order F–> Cl– > Br– > I–. This is because E-X bond length increases with an increase in size.
- Among Hexa halides, fluorides are the most stable because of steric reasons.
- Dihalides are sp3hybridized and so, are tetrahedral in shape.
- Hexafluorides are only stable halides that are gaseous and have sp3d2hybridization and an octahedral structure.
p-Block Elements Class 12 Notes
Dioxygen (O2)
(i) Preparation:
- By heating chlorates, nitrates and permanganates.
2 KClO3 → 2 KCl + 3O2 (laboratory method)
2 KMnO4 → K2MnO4 + MnO2 + O2
- By the thermal decomposition of the oxides of metals low in the electrochemical series and higher oxides of some.
2Ag2O(s) → 4Ag(s) + O2(g)
2Pb3O4(s) → 6PbO(s) + O2(g)
2HgO(s) → 2Hg(l) + O2(g)
2PbO2(s) → 2PbO(s) + O2(g) - By the decomposition of Hydrogen peroxide (H2O2) in presence of manganese dioxide. 2H2O2 → 2H2O + O2
- On large scale, it can be prepared from water or air. Electrolysis of water leads to the release of hydrogen at the cathode and oxygen at the anode. It is also obtained by the fractional distillation of air.
Properties of Oxygen:
- Colourless, odourless and tasteless gas.
- It is paramagnetic and exhibits allotropy.
- Three isotopes of oxygen are 168O, 178O and 188
- Oxygen does not burn but is a strong supporter of combustion.
- Dioxygen directly reacts with metals and non-metals(except with some metals like Au, Pt etc and with some noble gases). e.g.
2Ca + O2 → 2CaO
P4 + O2 → P4O10
4Al + 3O2 → 2Al2O3
C + O2 → CO2
Uses of Oxygen:
- Oxygen is used in oxyacetylene welding, in the manufacture of many metals, particularly steel.
- Oxygen cylinders are widely used in hospitals, high altitude flying and in
- Liquid O2is used in rocket fuels.
p-Block Elements Class 12 Notes
Ozone:
Ozone is an allotropic form of oxygen.
(i) Preparation: It is prepared by passing a silent electric discharge through pure and dry oxygen 10 – 15 % oxygen is converted to ozone.
3 O2(g)→ 2 O3(g); ∆H= +142 kJ/mol
Since the formation of ozone from oxygen is an endothermic process, a silent electric discharge should be used, unless the ozone formed undergoes decomposition.
(ii) Properties:
- Pure ozone is a pale blue gas.
- Dark blue liquid and violet-black
- Ozone has a characteristic smell.
- It is slightly soluble in water but more in turpentine oil, glacial acetic acid or CCl4.
- O3molecule is diamagnetic but O3– is paramagnetic.
- Ozone is thermodynamically unstable with respect to oxygen since its decomposition into oxygen results in the liberation of heat (∆H is negative) and an increase in entropy (∆S is positive). So the Gibbs energy change (∆G) for this process is always negative (∆G = ∆H – T∆S).
- Due to the ease with which it liberates nascent oxygen (O3→ O2 + O), it acts as a powerful oxidising agent. For e.g.,
- It oxidises lead sulphide to lead sulphate
- PbS(s) + 4O3(g) → PbSO4(s) +4O2(g)
- It oxidises H2S to S
- H2S + O3→ H2O + S ¯ (yellow)
Oxides of nitrogen (particularly nitric oxide) combine very rapidly with ozone and deplete it. Thus, nitrogen oxides emitted from the exhaust systems of supersonic jet aeroplanes, slowly depleting the concentration of the ozone layer in the upper atmosphere.
NO(g)+ O3(g) → NO2(g) + O2(g)
Bleaching Action:
O3 also bleaches coloured substances through oxidation.
O3 → O2 + [O]
Nascent Oxygen
Colouring Matter +[O] → Decolourised Matter
Tests for Ozone:
i) A filter paper soaked in alcoholic benzidine becomes brown when brought in contact with O3(this is not shown by H2O2)
ii)Tailing of mercury Pure mercury is a mobile liquid but when brought in contact with O3its mobility decreases and it starts sticking to glass surface forming a type of tail due to the dissolution of Hg2O (mercury sub-oxide) in Hg.
2Hg + O3 → Hg2O + O2
Estimation of Ozone: When ozone reacts with an excess of potassium iodide solution buffered with a borate buffer, iodine is liberated. The liberated iodine can be titrated against a standard solution of sodium thiosulphate. This is a quantitative method for estimating O3 gas.
Uses:
(i) It is used as a germicide, disinfectant and for sterilising water.
(ii) It is also used for bleaching oils, ivory, flour, starch, etc.
(iii) It acts as an oxidising agent in the manufacture of potassium permanganate.
(iv) For detecting the position of a double bond in the unsaturated organic compounds.
p-Block Elements Class 12 Notes
Sulphur its Compounds and Properties
Sulphur (S): Sulphur is the 2nd element of the oxygen family. Sulphur forms a large number of allotropes. Among these Yellow Rhombic (α-Sulphur) and Monoclinic (β -Sulphur) forms are the most important. The stable form at room temperature is rhombic sulphur, which transforms into monoclinic sulphur when heated above 369 K.
(i) Rhombic Sulphur (a – Sulphur): This allotrope is yellow in colour, m.p. 385.8 K and specific gravity 2.06. Rhombic sulphur crystals are formed on evaporating the solution of roll sulphur in CS2. It is insoluble in water but dissolved to some extent in benzene, alcohol and ether. It is readily soluble in CS2.
(ii) Monoclinic (β -Sulphur): It is prepared by melting rhombic sulphur in a dish and cooling, till a crust is formed. Two holes are made in the crust and the remaining liquid is poured out. On removing the crust, colourless needle-shaped crystals of β-sulphur are formed. It is stable above 369 K and transforms into α-sulphur below it. At 369 K both the forms are stable. This temperature is called transition temperature.
Both rhombic and monoclinic sulphur have S8 molecules. The S8 ring in both forms is puckered and has a crown shape.
(i) Preparation:
Sulphur dioxide is formed when sulphur is burnt in air or oxygen: S(s) + O2(g) → SO2 (g)
In the laboratory, it is obtained by treating a sulphite with dilute sulphuric acid.
SO32-(aq) + 2H+ (aq) → H2O(l) + SO2 (g)
Industrially, it is produced by roasting of sulphide ores.
4 FeS2(s) + 11 O2(g) → 2 Fe2O3(s) + 8 SO2(g)
(ii) Properties:
- Sulphur dioxide is a colourless gas with a pungent smell and is highly soluble in water.
- With water, it forms a solution of sulphurous acid which is a dibasic acid and forms two types of salts with alkalies – normal salt (sulphite) and acid salt (bisulphate or hydrogen sulphite).
SO2(g) + H2O(l) → H2SO3(aq) - Neither burns nor helps in burning but burning magnesium and potassium continue to burn in its atmosphere.
3Mg + SO2 → 2 MgO + MgS
4K + 3SO2 → K2SO3 + K2S2O3
- With sodium hydroxide solution, it forms sodium sulphite, which then reacts with more sulphur dioxide to form sodium hydrogen sulphite.
2NaOH + SO2 → Na2SO3 + H2O
Na2SO3 + H2O + SO2 → 2NaHSO3
- SO2is oxidised to sulphur trioxide by oxygen in the presence of vanadium pentoxide (V2O5) catalyst. 2SO2 + O2 → 2SO3
- Moist sulphur dioxide behaves as a reducing agent. It converts iron (III) ions to iron (II) ions and decolourises acidified potassium permanganate(VII) solution (This used as a test for SO2).
2Fe3+ + SO2 + 2H2O → 2Fe2+ + SO42- + 4H+
5 SO2 + 2MnO4– + 2H2O → 5 SO42- + 4H+ + 2Mn2+
Acidified K2Cr2O7 → Cr3+ (green coloured solution)
Bleaching Action: SO2 + 2H2O → H2SO4 + 2H
This is due to the reducing nature of SO2
Coloured matter + H ↔ Colourless matter. Therefore, the bleaching is temporary.
Uses: Sulphur dioxide is used:
- Used in the manufacture of H2SO4& paper from wood pulp.
- As a bleaching agent for delicate articles like wool, silk and straw.
- Used in the refining of petroleum and sugar.
- Liquid SO2is used as a solvent to dissolve a number of organic and inorganic chemicals.
p-Block Elements Class 12 Notes
Oxoacids of Sulphur: Sulphur forms a large no. of oxoacids. Sulphurous acid (H2SO3), Sulphuric acid (H2SO4), Dithionic acid (H2S2O6), Peroxomonosulphuric acid (Caro’s acid, H2SO5), Peroxodisulphuric acid (Marshell’s acid, H2S2O8) etc. The structure of some oxoacids are
Sulphuric acid (H2SO4): One of the most important oxoacids of sulphur is H2SO4. It is also known as the king of all acid.
- Manufacture: Sulphuric acid is manufactured by the Contact Process which involves three steps:
- Manufacture: Sulphuric acid is manufactured by the Contact Process which involves three steps:
Step 1: Burning of sulphur or sulphide ores in the air to generate SO2.
S(s) + O2(g) → SO2 (g)
OR
4FeS2(s) + 11 O2(g) → 2 Fe2O3(s) + 8 SO2(g)
Step 2: Conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5).
2SO2 + O2 → 2SO3
Step 3: Absorption of SO3 in H2SO4 to give Oleum (H2S2O7).
SO3 + H2SO4 → H2S2O7
Dilution of oleum with water gives H2SO4 of the desired concentration.
H2S2O7 + H2O → 2H2SO4.
Lead Chamber Process (Industrial method):
2SO2 + O2 (air) + 2H2O + [NO] (catalyst) ¾® 2H2SO4 + [NO] (catalyst)
Acid obtained is 80% pure and is known as Brown Oil of Vitriol.
Properties of Sulphuric acid:
- Sulphuric acid is a colourless, dense, oily liquid.
- It dissolves in water with the evolution of a large quantity of heat. Hence, for diluting the acid, the concentrated acid must be added slowly into the water with constant stirring.
- It fumes strongly in moist air and is highly corrosive in nature.
- Thermal decomposition: It decomposes at 440 0C
H2SO4 ↔H2O+SO3
- The chemical reactions of sulphuric acid are due to
(i) Its low volatility
(ii) Strong acidic character
(iii) Strong affinity for water and
(iv) Its ability to act as an oxidising agent.
In an aqueous solution, sulphuric acid ionises in two steps.
H2SO4(aq) + H2O(l) → H3O+(aq) + HSO4–
HSO4–(aq) + H2O(l) → H3O+(aq) + SO42-
So, it is dibasic and forms two series of salts: Normal Sulphates and Acid
Sulphates.
- Decomposes carbonates and bicarbonates into carbon dioxide
Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2
NaHCO3 + H2SO4 → NaHSO4 + H2O + CO2
Concentrated sulphuric acid is a strong dehydrating agent and drying agent. Many wet gases can be dried by passing them through sulphuric acid. Sulphuric acid removes water from organic compounds
e.g.: C12H22O11 + H2SO4 → 12C + 11H2O
- Hot concentrated sulphuric acid is a moderately strong oxidising agent. It oxidises both metals and non-metals and the acid itself reduces to SO2.
Cu + 2 H2SO4(conc.) → CuSO4 + SO 2 + 2H2O
S + 2H 2SO4(conc.) → 3SO2 + 2H2O
C + 2H2SO4(conc.) → CO2 + 2 SO2 + 2 H2O
Uses: The important uses of Sulphuric acid are:
- In the manufacture of fertilizers
- In petroleum refining
- In the manufacture of pigments, paints and dyestuff intermediates.
- In the detergent industry
- In metallurgical applications
- As electrolyte in storage batteries.
- In the manufacture of nitrocellulose products and
- As a laboratory
Group 17 Elements and Propertiesp-Block Elements Class 12 Notes
Group 17 Elements
Group 17 includes Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are collectively known as the halogens (Greek halo means salt and genes born i.e., salt producers). The halogens are highly reactive non-metallic elements.
(i) Electronic Configuration: All these elements have seven electrons in their outermost shell (ns2 np5) which is one electron short of the next noble gas.
(ii) Atomic and ionic radii: Halogens have the smallest atomic radii in their respective periods because of the maximum effective nuclear charge. Atomic and ionic radii increase from fluorine to iodine due to the increasing number of shells.
(iii) Ionisation Enthalpy: They have very high ionization enthalpy because of their small size as compared to other groups. Due to an increase in atomic size, ionization enthalpy decreases down the group.
(iv) Electron Gain Enthalpy:
a) Halogens have maximum negative electron gain enthalpy because these elements have only one electron less than stable noble gas configuration.
b) Electron gain enthalpy becomes less negative down the group because atomic size increases down the group.
c) The negative electron gain enthalpy of fluorine is less than that of chlorine. It is because, in fluorine, the incoming electron goes to the 2p subshell, but in Cl, it enters into the 3p subshell. Due to the compactness of 2p subshell compared to 3p subshell, the electron-electron repulsion is greater in fluorine than in chlorine. So, F does not easily gain electrons.(v) Electronegativity: They have very high electronegativity. The electronegativity decreases down the group. Fluorine is the most electronegative element in the periodic table.
(vi) Bond Dissociation Enthalpy:
a) Bond dissociation enthalpy follows the order: Cl2> Br2> F2 > I2
b) This is because as the size increases bond length increases.
c) Bond dissociation enthalpy of Cl2is more than F2because there are large electronic repulsions of lone pairs present in F2.(vii) Colour: All halogens are colored because of absorption of radiations in the visible region which results in the excitation of outer electrons to higher energy levels.(viii) Oxidising power:
a) All halogens are strong oxidizing agents because they have a strong tendency to accept electrons.
b) Order of oxidizing power is: F2> Cl2> Br2 > I2(ix) Physical Properties: Fluorine and chlorine are gases, bromine is a liquid and iodine is a solid. Their melting and boiling points steadily increase with atomic number.
(x) Oxidation State:
a) All the halogens exhibit –1 oxidation state. However, chlorine, bromine and iodine exhibit + 1, + 3, + 5 and + 7oxidation states also.
b) The higher oxidation states of chlorine, bromine, and iodine are realized mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms., in interhalogens, oxides, and oxoacids.
c) The fluorine atom has no d orbitals in its valence shell and therefore cannot expand its octet. Being the most electronegative, it exhibits only – 1 oxidation state.
d) The ready acceptance of an electron is the reason for the strong oxidizing nature of halogens. F2is the strongest oxidizing halogen and it oxidizes other halide ions in solution or even in the solid phase.
e) Fluorine oxidizes water to oxygen whereas chlorine and bromine react with water to form corresponding hydrohalic and hypohalous acids.
2 F2 (g) + 2 H2O(l) → 4 H+ (aq) + 4 F– (aq) + O2 (g)
X2 (g) + H2O (l) → HX(aq) + HOX (aq) (Where X = Cl or Br)f) More the value of the SRP, the more powerful is the (algebraically) oxidizing agent.Hence the order of oxidising power is F2 > Cl2 > Br2 > I2. Since Standard Reduction Potential is the highest for F2, it is the strongest oxidizing agent.Anomalous Behavior of Fluorine:
(i) Due to the small size.
(ii) Highest Electronegativity.
(iii) Low F-F bond dissociation enthalpy.
(iv) Non-availability of d – orbitals in the valence shell.
(v) Most of the reactions of fluorine are exothermic.
(vi) F forms only one oxoacid while other halogens form a number of oxoacids.
(vii) Ionic and covalent radii, melting point and boiling point and electron gain enthalpy are quite lower than expected.
(viii) Hydrogen fluoride is a liquid due to strong hydrogen bonding. While the hydrogen halides of other elements are gases.Reactivity towards Hydrogen: They all react with hydrogen to give hydrogen halides but the affinity for hydrogen decreases from fluorine to iodine. They dissolve in water to form hydrohalic acids.
The acidic strength of these acids varies in the order: HF < HCl < HBr < HI. The stability of these halides decreases down the group this is due to a decrease in bond (H–X) dissociation enthalpy in the order H – F > H – Cl > H –Br > H – I.
HF has strong intermolecular H bonding.
% Ionic character: HF > HCl > HBr > HI.
Dipole moment HF > HCl > HBr > HI.
Electronegativity decreases down the group.
Reducing power: HF < HCl < HBr < HI.
As the size increases, van der Waals forces increase, and hence boiling point increases.Reactivity towards Oxygen:
(i) Halogens form many oxides with oxygen but most of them are unstable.
(ii) Fluorine forms two oxides OF2 and O2F2. However, only OF2 is thermally stable at 298 K. These oxides are essentially oxygen fluorides because of the higher electronegativity of fluorine than oxygen.
Both are strong fluorinating agents. O2F2 oxidizes plutonium to PuF6 and the reactions are used in removing plutonium as PuF6 from spent nuclear fuel.
(iii) Chlorine, bromine, and iodine form oxides in which the oxidation states of these halogen ranges from + 1 to + 7. The higher oxides of halogens tend to be more stable than the lower ones.
(iv) Chlorine oxides, Cl2O, ClO2, Cl2O6, and Cl2O7 are highly reactive oxidizing agents and tend to explode. ClO2 is used as a bleaching agent for paper pulp and textiles and in water treatment.
(v) The bromine oxides, Br2O, BrO2, BrO3 are the least stable halogen oxides and exist only at low temperatures. They are very powerful oxidizing agents.
(vi) The iodine oxides, I2O4, I2O5, I2O7 are insoluble solids and decompose on heating. I2O5 is a very good oxidizing agent and is used in the estimation of carbon monoxide.Reactivity towards metals: Halogen reacts with metals to form metal halides. For e.g., bromine reacts with magnesium to give magnesium bromide.
Ionic character: MF > MCl > MBr > MI. Halides in a higher oxidation state will be more covalent than the ones in a lower oxidation state.
Interhalogen Compounds: Reactivity of Halogens Towards other Halogens: Halogens combine amongst themselves to form a number of compounds known as interhalogen of the types X X’, X X’3, X X’5, and X X’7. Where X is a larger size halogen and X’ is a smaller size halogen.
Chlorine its Properties and Oxoacids of Chlorine
Chlorine (Cl2)
(1) Preparation: It can be prepared by any one of the following methods:
(i) By heating manganese dioxide with concentrated hydrochloric acid.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Conc. HCl can be replaced by a mixture of common salt and concentrated H2SO4
4NaCl + MnO2 + 4H2SO4 → MnCl2+ 4NaHSO4 + 2H2O + Cl2
(ii) By the action of HCl on potassium permanganate.
2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2(2) Manufacture of Chlorine:
(i) Deacon’s Process:
By oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 (catalyst) at 723 K.
4HCl+O2 ⎯⎯⎯⎯→2Cl2 +2H2O
(ii) Electrolytic Process: Chlorine is obtained by the electrolysis of brine solution (concentrated NaCl solution). During electrolysis, chlorine is liberated at the anode.
(i) It is a greenish-yellow gas with a pungent and suffocating odour.
(ii) It is soluble in water.
(iii) It reacts with a number of metals and non-metals to form chlorides.
2Al + 3Cl2 → 2AlCl3
P4 + 6Cl2 → 4PCl3
2Na + Cl2 → 2NaCl
S8 + 4Cl2 → 4S2Cl2
2Fe + 3Cl2 → 2FeCl3
(iv) With excess ammonia, chlorine gives nitrogen and ammonium chloride whereas with excess chlorine, nitrogen trichloride(explosive) is formed.
8NH3 + 3Cl2 → 6NH4Cl + N2
(excess)
NH3 + 3Cl2 → NCl3 + 3HCl
(excess)
(v) With cold and dilute alkalies chlorine produces a mixture of chloride and hypochlorite.
2NaOH + Cl2 → NaCl + NaOCl + H2O
(cold and dilute)
With hot and concentrated alkalies it gives chloride and chlorate.
6 NaOH + 3Cl2 → 5NaCl + NaClO3 + 3H2O
(hot and conc.)
(vi) With dry slaked lime it gives bleaching powder-+.
2Ca(OH)2 + 2Cl2 → CaOCl2 + CaCl2 + 2H2O
(vii) Chlorine reacts with hydrocarbons and gives substitution products with saturated hydrocarbons and addition products with unsaturated hydrocarbons.
CH4 + Cl2 ⎯⎯UV⎯→ CH3Cl + HCl
Methane Methyl chloride
(viii) Chlorine water on standing loses its yellow colour due to the formation of HCl and HOCl. Hypochlorous acid (HOCl)so formed is unstable and dissociates to give nascent oxygen which is responsible for oxidising and bleaching properties of chlorine.
(ix) It oxidises ferrous to ferric, sulphite to sulphate, sulphur dioxide to sulphuric acid and iodine to iodic acid.
2FeSO4 + H2SO4 + Cl2 → Fe2(SO4)3 + 2HCl
Na2SO3 + Cl2 + H2O → Na2SO4 + 2HCl
SO2 + 2H2O + Cl2 → H2SO4 + 2HCl
I2 + 6H2O + 5Cl2 → 2HIO3 + 10HCl
(x) It is a powerful bleaching agent. Bleaching action is due to oxidation.
Cl2 + H2O → 2HCl + [O]
Coloured substance + [O] → Colourless substance
It bleaches vegetable or organic matter in the presence of moisture. Its bleaching action is permanent.(4) Uses of Cl2: It is used
(i) For bleaching wood pulp, bleaching cotton and textiles
(ii) In the extraction of gold and platinum
(iii) In the manufacture of dyes, drugs and organic compounds such as CCl4, CHCl3, DDT, refrigerants, etc.
(iv) In sterilizing drinking water
(v) Preparation of poisonous gases such as Phosgene (COCl2), Tear gas (CCl3NO2), Mustard gas (ClCH2CH2SCH2CH2Cl).Hydrochloric Acid (HCl)
(1) Preparation: It is prepared in the laboratory, by heating sodium chloride with concentrated sulphuric acid.
NaCl + H2SO4 ⎯⎯420⎯K⎯→ NaHSO4 + HCl
NaHSO4 + NaCl ⎯⎯823⎯K⎯→ Na2SO4 + HCl
(2) Properties:
(i) These are colourless, pungent-smelling gas with acidic tastes.
(ii) It is heavier than air, can be liquified to colourless liquids.
(iii) These are neither combustible nor supporter of combustion.
(iv) When perfectly dry, they have no action on litmus, but in presence of moisture, they turn blue litmus red, showing acidic nature. Among HX, HI is the strongest and HF is the weakest acid.
(v) These are quite soluble in water. HCl ionises as below.
HCl(g) + H2O (l) ® H3O+ (aq) + Cl– (aq) Ka = 107.
(vi) Its aqueous solution is called hydrochloric acid. The high value of the dissociation constant (Ka) indicates that it is a strong acid in water.
(vii) It reacts with NH3and gives white fumes of NH4Cl.
NH3 + HCl → NH4Cl
(viii) When three parts of concentrated HCl and one part of concentrated HNO3are mixed, aqua regia is formed which is used for dissolving noble metals, e.g., gold, platinum.
Au + 4 H+ + NO3– + 4Cl– →AuCl4– + NO + 2H2O
3Pt + 16H++ 4NO3– + 18Cl– → 3PtCl62- + 4NO + 8H2O
(ix) Hydrochloric acid decomposes salts of weaker acids like carbonates, hydrogen carbonates, sulphites, etc.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
NaHCO3 + HCl → NaCl + H2O + CO2
Na2SO3 + 2HCl → 2NaCl + H2O + SO2
(x) Reaction with metals oxides, hydroxides and bicarbonates
Zn + 2HCl → ZnCl2 + H2
MgO + 2HCl → MgCl2 + H2O
NaOH + HCl → NaCl + H2O
Detection of cation: HCl:
AgNO3 + HCl → AgCl¯ (white) + HNO3
(CH3COO)2Pb + 2HCl → PbCl2¯ (white) + 2CH3COOH
Hg(NO3)2 + 2HCl → Hg2Cl2 ¯ (white) + 2HNO3(3) Uses: It is used
(i) In the manufacture of chlorine, NH4Cl and glucose (from corn starch).
(ii) For extracting glue from bones and purifying boneblack,
(iii) In medicine and as a laboratory
(iv) HI is used as a reducing agent in organic chemistry.Oxoacids of Halogens
The term oxoacid now refers to a compound that contains oxygen, at least one other element, and at least one hydrogen bound to oxygen.
(i) Fluorine forms only one oxoacid HOF (Fluoric (I) acid or hypofluorous acid)due to high electronegativity.
(ii) The other halogens form several oxoacids like Hypohalous acid (HOX), halous acid (HOXO), halic acid (HOXO2) and perhalic acid (HOXO3). They are stable only in aqueous solutions or in the form of their salts.
(iii) Chlorine forms 4 types of oxoacids –hypochlorous acid (HOCl), Chlorous acid (HOClO or HClO2), Choric acid (HOClO2 or HClO3) and perchloric acid (HOClO3 or HClO4). The structures of them are:
Reason: HClO4 → H+ + ClO4– -most stable
(v) Acid strength: HOF > HOCl > HOBr > HOI
This is because Fluorine is the most electronegative element.p-Block Elements Class 12 Notes
Noble Gases (Group 18) Elements
Group 18 Elements
Group 18 consists of six elements- helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). All these are gases and chemically unreactive. They form very few compounds. Because of this, they are termed noble gases.(i) Occurrence: All the noble gases except radon occur in the atmosphere. Their atmospheric abundance in dry air is ~ 1% by volume of which argon is the major constituent. Helium and sometimes neon is found in minerals of radioactive origin e.g., pitchblende, monazite, cleveite. The main commercial source of helium is natural gas. Xenon and radon are the rarest elements of the group.
The most abundant element in the air is Ar. Order of abundance in the air is Ar > Ne > Kr > He > Xe.
(ii) Electronic Configuration: All noble gases have general electronic configuration ns2np6 except helium which has 1s2. Many of the properties of noble gases including their inactive nature are ascribed to their closed-shell structures.
(iii) Ionisation Enthalpy: They have very high ionization enthalpy because of completely filled orbitals. Ionization enthalpy decreases down the group because of an increase in size.
(iv) Atomic radii: Increases down the group because the number of shells increases down the group.
(v) Electron Gain Enthalpy: Since noble gases have stable electronic configurations, they have no tendency to accept the electron and therefore, have larger positive values of electron gain enthalpy.
(vi) Melting and boiling point: Low melting and boiling point because only weak dispersion forces are present.
(vii) Chemical Properties: The reactivity of noble gases has been investigated occasionally ever since their discovery, but all attempts to force them to react to form the compounds were unsuccessful for quite a few years. In March 1962, Neil Bartlett, then at the University of British Columbia, observed the reaction of a noble gas. First, he prepared a red compound which is formulated as O2+ PtF6 –. He then realized that the first ionization enthalpy of molecular oxygen (1175 kJ mol–1) was almost identical to that of xenon (1170 kJ mol–1). He made efforts to prepare the same type of compound with Xe+PtF6– by mixing PtF6 and Xenon. After this discovery, a number of xenon compounds mainly with the most electronegative elements like fluorine and oxygen, have been synthesized.
(viii) Xenon-fluorine compounds: Xenon forms three binary fluorides, XeF2, XeF4 and XeF6 by the direct reaction of elements under suitable conditions.
Xe (g) + F2 (g) 673K, 1 bar XeF2(s)
(xenon in excess) →
Xe (g) + 2F2 (g) 873K, 7 bar XeF4(s)
(1:5 ratio) →
Xe (g) + 3F2 (g) 573K, 60-70bar XeF6(s)
(1:20 ratio) →
XeF6 can also be prepared by the interaction of XeF4 and O2F2 at 143K.
XeF4 +O2F2 →XeF6 +O2
XeF2, XeF4 and XeF6 are colourless crystalline solids.
They are powerful fluorinating agents.
They are readily hydrolyzed even by traces of water. For example, XeF2 is hydrolyzed to give Xe, HF, and O2.
2XeF2 (s) + 2H2O (l) → 2Xe (g) + 4 HF(aq) + O2(g)(ix) Structures: XeF2 and XeF4 have linear and square planar structures respectively. XeF6 has seven electron pairs (6 bonding pairs and one lone pair) and thus, have a distorted octahedral structure.
(a) XeO3: It is obtained by the hydrolysis of XeF4and XeF6 with water.
6XeF4 + 12 H2O → 4Xe + 2XeO3 + 24 HF + 3 O2
XeF6 + 3 H2O → XeO3 + 6 HF
(b) XeOF2andXeOF4: Partial hydrolysis of XeF6 gives oxyfluorides, XeOF4 and XeO2F2.
XeF6 + H2O → XeOF4 + 2 HF
XeF6 + 2 H2O → XeO2F2 + 4HF
XeO3 is a colourless explosive solid and has a pyramidal molecular structure. XeOF4 is a colourless volatile liquid and has a square pyramidal molecular structure.
(a) Helium is used in filling balloons for meteorological observations. It is also used in gas-cooled nuclear reactors. Liquid helium is used as a cryogenic agent for carrying out various experiments at low temperatures. It is used as a diluent for oxygen in modern diving apparatus because of its very low solubility in blood.
(b) Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. Neon bulbs are used in botanical gardens and in greenhouses.
(c) Argon is used to provide an inert atmosphere in high-temperature metallurgical processes and for filling electric bulbs. It is also used in the laboratory for handling substances that are air-sensitive.
(d) Xenon and Krypton are used in light bulbs designed for special purposes.