Practice Questions p-Block Elements
Practice Questions p-Block Elements
1. Why is N2 less reactive at room temperature?
2. Mention the condition required to maximize the yield of ammonia.
3. Why nitric oxide becomes brown when released in air?
4. Nitrogen shows little tendency of catenation.
5. What is the covalence of nitrogen in N2O5?
6. What happens when white phosphorus is heated with conc. NaOH solution in an inert atmosphere of CO2?
7. Why HF is not stored in glass bottles?
8. Write the important points to show anomalous behaviour of nitrogen in group 15.
9. Write the possible oxidation states of N in their compounds along with suitable examples.
10. What happens when H3PO3 is heated?
11. Nitrogen cannot expand its covalency beyond 4 why?
12. Why is NH3 a good complexing agent?
13. How is phosphine prepared from white phosphorus?
14. Give reason:
(a) SO2 acts as both oxidizing and reducing agents but SO3 only as an oxidizing agent.
(b) Oxygen shows covalency of two while sulphur shows covalency up to six.
15. What happens when?
(a) SO3 is absorbed in H2SO4?
(b) Potassium chlorate is heated?
(c) HCl is reacted with O2 in presence of CuCl2?
16. Cu dissolves in HNO3 but not HCl. Why?
17. Write a chemical reaction to show the dehydrating property of sulphuric acid.
18. How would you account for the following? The elections gain enthalpy with a negative sign is less for oxygen than that of sulphur.
19. SF4 is easily hydrolyzed whereas SF6 is not. Why?
20. Why does ozone act as a powerful oxidizing agent?
21. Write the conditions to maximize the yield of sulphuric acid by contact process.
22. (a) Why the yellow colour of chlorine water fades on standing?
(b) Write the equation when Cl2 is passed through cold and dil. NaOH.
23. What are oxygen fluorides? Give example and oxidation state of oxygen in it. Why are they not called oxides?
24. Complete the reaction (a) Ca(OH)2 + Cl2 ⎯⎯→
(b) CH4 + Cl2 ⎯⎯⎯UV→
(c) MnO2 + HCl ⎯⎯→
25. How is SO2 an air pollutant?
26. Tendency to show -2 oxidation states diminishes from S to Po. Why?
27. Which form of sulphur is paramagnetic in nature?
28. Arrange group 15 hydrides in the correct order of stability, bond angle, bond dissociation enthalpy, reducing character and basicity.
29. Write the following chemical reactions
(a) Reaction of Cu with dilute HNO3.
(b) Reaction of Cu with concentrated HNO3.
(c) Reaction of Zn with dilute HNO3.
(d) Reaction of calcium phosphide with H2O.
(e) Reaction of white phosphorus with an excess of Cl2.
30. With what neutral molecules are ClO– is electronic? Is that molecule a Lewis base?
31. Why are halogens coloured?
32. Give reason
(i) Bleaching of the flower by Cl2 is permanent while SO2 is temporary.
(ii) pKa value for HOCl is higher than that of HOCIO
(iii) ICI is more relative than l2.
33. What are interhalogen compounds? Give the equation for the preparation of ClF3, ICl. What happens when they are hydrolyzed?
34. Complete the following reaction:
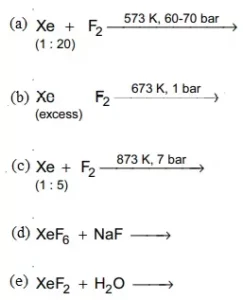
35. On heating lead (II) nitrate gives a brown gas ‘A’ the gas ‘A’ on cooling changes to colorless solid ‘B’ solid ‘B’ on heating with NO changes to blue solid ‘C’ identify A, B, and C also write reactions involved and draw the structures of ‘B’ and ‘C’.
Practice Questions p-Block Elements