Practice Questions (Answers) p-Block Elements
Practice Questions (Answers) p-Block Elements
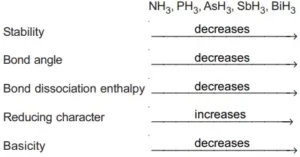
Ans 1. N2 is less reactive at room temperature because the bond enthalpy of the triple bond between N ≡ N is very high.
Ans 2. (i) Low temperature (700 K), High Pressure (200 atm).
(ii) Use of catalysts such as iron oxide along with Mo or K2O and Al2O3 promoter.
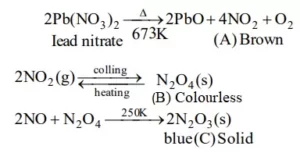
Ans 3. 2 NO + O2 → 2 NO2
Colourless Brown
Ans 4. N−N single bond is weak as nitrogen has small size, the lone pair of electrons on two nitrogen repel each other
Ans 5. Since the nitrogen atom has 4 shared electron pairs, the covalence of nitrogen is 4.
Ans 6. White phosphorus dissolves in boiling NaOH producing phosphine gas and sodium hypophosphite.
P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3
Ans 7. HF attacks sodium silicate which is the main constituent of glass. As a result, the glass bottles are slowly corroded or eaten up. Therefore, HF cannot be stored in glass bottles. It is normally kept in wax bottles.
Na2SiO3 + 6 HF → Na2SiF6 + 3 H2O
Ans 8. (a) Nitrogen is a diatomic molecule and inert.
(b) It shows a weaker catenation tendency.
(c) Maximum covalency of 4 only
(d) Forms pπ-pπ bond.
Ans 9. Following are the compounds of nitrogen along with oxidation state

Ans 10. H3PO3 → H3PO4 + PH3
Phosphorous acid Phosphoric acid Phosphine
Ans 11. Nitrogen does not have a vacant d-orbital to accommodate unpaired s-electron.
Ans 12. Ammonia is a good complexing agent because nitrogen atom has a lone pair of electrons that can easily be donated to form a coordinate bond.
Ans 13. P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
When pure it is non-inflammable but explodes in presence of P2H4 or Cl2. It is colourless. Used in Holme’s signal, smokescreen
Ans 14. (a) In SO2, S has oxidation number +4. It can both increase and decrease its oxidation number. But SO2 has S in +6 state, hence, during reaction oxidation number decrease and act as an oxidising agent.
(b) Oxygen has a small size and do not have a vacant d orbital, while sulphur has a larger size.
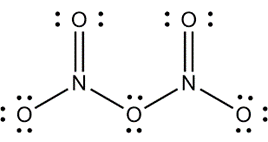
Ans 15. (a) SO3 + H2SO4 → H2S2O7
(b) KClO3 → KCl + O2
(c) HCl + O2 ——CuCl2→ 2Cl2 + 2H2O
Practice Questions (Answers) p-Block Elements
Ans 16. Copper is placed below H in electrochemical series and does not liberate H2 from acid. However nitric acid being strong oxidant oxidises Cu.
Ans 17. On heating sugar in presence of sulphuric acid, we get
C6H12O6 → 6C + 6H2O
Sulphuric acid reacts with glucose to produce carbon and hydrated sulphuric acid.
6H2SO4 + C6H12O6 ⟶ 6C + 6(H2SO4.H2O)
Ans 18. This is due to the smaller size of oxygen the electron cloud is distributed over a small region of space making electron density high which repels the incoming electrons.
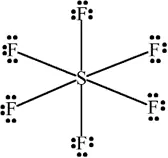
Ans 19. S atom in SF4 is not satirically protected as it is surrounded by only four F atoms, so the attack of H2O molecules can take place easily and hence hydrolysis occurs easily in contrast. In SF6 S is sterically protected by six F atoms. Therefore, does not allow H2O molecules to attack S atoms. As a result of this SF6 does not undergo hydrolysis.
Ans 20. Because ozone liberates nascent oxygen very easily
O3 → O2 + [O]
Ans 21. Following are the conditions to maximise the yield of Sulphuric acid
(a) The presence of catalyst V2O5
(b) High pressure
(c) Low temperature
Ans 22. (a) Cl2 + H2O → HCl + HClO
Yellow and Highly Unstable
HClO → HCl (Colorless) + [O]
(b) Cl2 + NaOH → NaCl + NaClO + H2O
(Hot & Conc.)
Ans 23. Compounds of oxygen and fluorine are oxygen fluorides e.g., OF2 and O2F2. In both compounds oxygen exhibits +2 and +1 oxidation states respectively. As fluorine is more electronegative than oxygen, so compounds are called as fluorides not oxide
Ans 24. (a) Ca(OH)2 → Ca(OCl)2 + CaCl2 + H2O
(b) CH4 + Cl2 → CH3Cl + HCl
(c) MnO2 + HCl → MnCl2 + Cl2 + H2O
Ans 25. SO2 present in the atmosphere dissolves in rainwater to form Sulfurous acid(H2SO3). The Sulfurous acid destroys buildings. SO2 inhibits plant growth and causes eye irritation in humans.
Practice Questions (Answers) p-Block Elements
Ans 26. It is because of the increase in atomic size from S to Po, tendency to attract electrons i.e Electronegativity decreases.
Ans 27. In the vapour phase, sulphur exists in S2 molecule which has two unpaired electrons in the anti-boding molecular orbital like O2 and hence paramagnetic in nature.
Ans 29. (a) Cu + HNO3 (dil) → Cu(NO3)2 + NO + H2O
(b) Cu + HNO3 (Conc.) → Cu(NO3)2 + NO2 + H2O
(c) Zn + HNO3 (dil.) → Zn(NO3)2 + N2O + H2O
(d) Ca3P2 + H2O → Ca(OH)2 + PH3 (Phosphine)
(e) P4 (white) + 10Cl2 (excess) → 4PCl5
Ans 30. ClO– has 26 electrons it is isoelectronic with two natural molecules. These are oxygen difluoride (OF2) and chlorine fluoride (CIF). Out of these CIF is a Lewis base as it can further donate electrons to more atoms of fluorine.
Ans 31. The colour of halogens is due to the absorption of light in the visible region as a result electrons get excited to higher energy levels and colours are seen.
Ans 32. (i) Chlorine bleaches Coloured material by oxidation:
Cl2 + H2O → 2 HCl + [O]
Coloured +[O]→ Colourless material + [O] → Colourless material
Hence, the bleaching is permanent.
Whereas, sulphur dioxide, SO2 bleach coloured material by reduction and hence bleaching is temporary. When the bleached colourless material is exposed to air, it gets oxidised and the colour is restored.
(ii) In HOClO, chlorine is in higher oxidation (+3) state than in HOCl (+1), therefore HOClO is a stronger acid than HOCl.
(iii) ICl is more reactive than I2 because the I – Cl bond is weaker than the I – I bond. A bond between two different atoms is always weaker than that between the same atoms. Consequently, ICl breaks easily to form individual halogen atoms that readily bring out the reactions.
Ans 33. When halogen combines with other halogens it forms the interhalogen compound, of type XX’, XX3’, XX5’, XX7’. Where the size of halogen X is larger than X’.
Preparation of ClF3 : Cl2 + 3 F2 (excess)—573K→ ClF3
I2 + Cl2 → ICl
On hydrolysis: XX’ + H2O → HX’ + HOX
Ans 34. (a) XeF6
(b) XeF2
(c) XeF4
(d) Na+ [XeF7]–
(e) Xe + Hf + O2
Practice Questions (Answers) p-Block Elements