Practice Questions (Answers) Chapter 11 Class 12 Chemistry
Practice Questions (Answers) Chapter 11 Class 12 Chemistry
Ans 1. It is due to the poor intermolecular force of attraction.
Ans 2. The lone pair on the methoxy group oxygen is more available for participating in resonance into the ring than the lone pair on hydroxyl oxygen due to the +I effect of the methyl group.
Ans 3. Ionization of alcohol is represented by
ROH + H2O ↔ RO– + H3O+
the negative charge in the alkoxide ion is localized on the oxygen atom. In the case of phenoxide ion, the negative charge on oxygen undergoes delocalization that makes it more stable as shown below
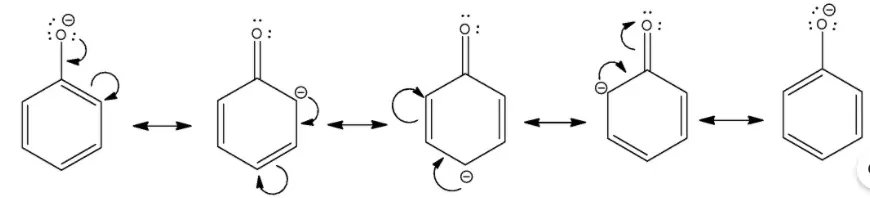
Ans 4. This is because in p-nitrophenol, there is no hydrogen bonding and hence reacts with bases like NaHCO3 which is a weaker base. Phenol is less acidic due to the presence of the polar O-H group.
Since oxygen is more electronegative than hydrogen, the electron pair of O-H bond is pulled more towards O and H+ ion is easily released in an aqueous solution.
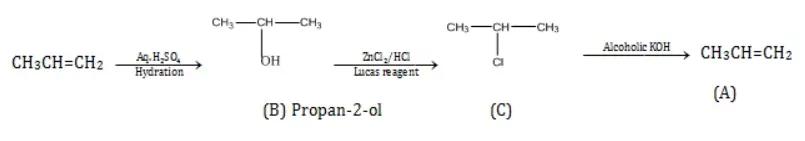
Ans 5. Primary alcohol on reacting with Luca’s reagent (which is equimolar ZnCl2 and concentrated HCl) gives no turbidity.
Whereas adding Luca’s reagent to secondary alcohols produces turbidity in the solution within 5 minutes,
The addition of Lucas reagent in tertiary alcohols produces instant turbidity.
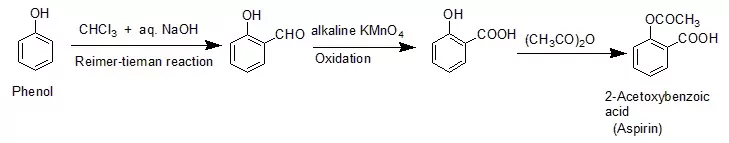
Ans 6. Reimer- Tieman Reaction
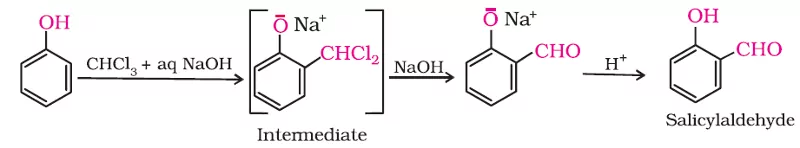
Due to intra-molecular H-bounding
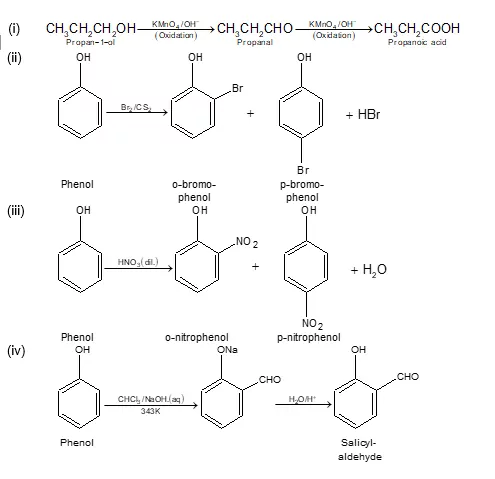
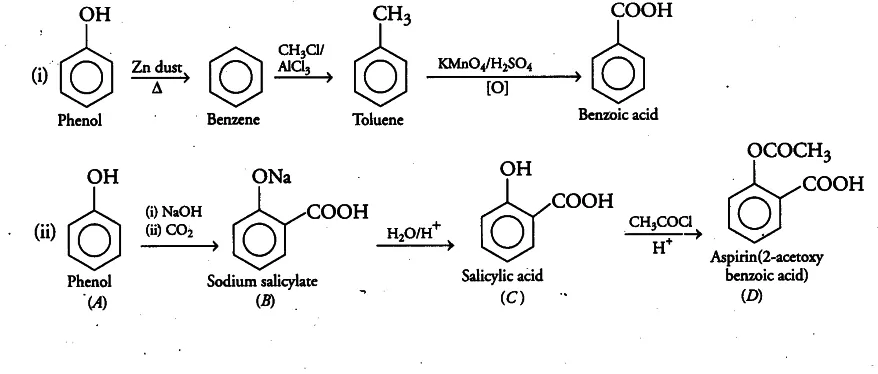
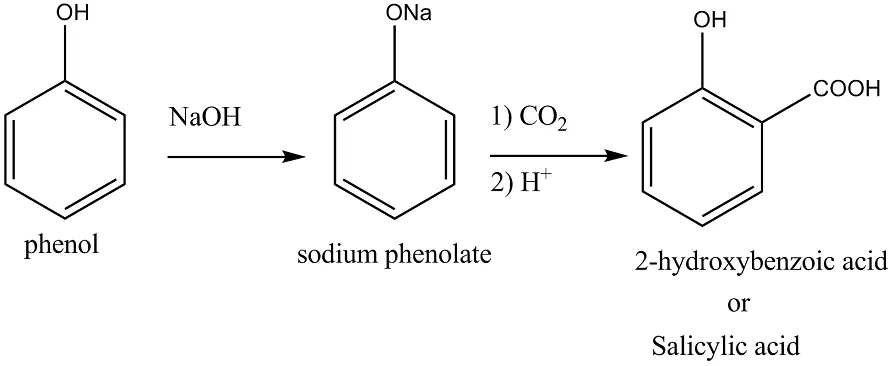
Ans 7. When phenol is treated with sodium hydroxide, sodium phenoxide is produced. This sodium phenoxide when treated with carbon dioxide, followed by acidification, undergoes electrophilic substitution to give ortho-hydroxybenzoic acid as the main product. This reaction is known as Kolbe’s reaction.
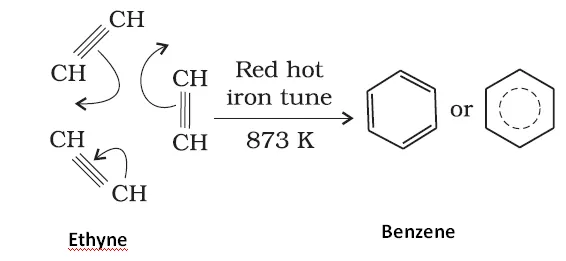
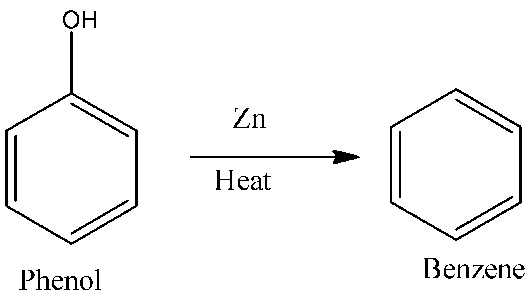
Ans 8. Conversion of phenol to benzene
Ans 10. Friedel-Craft Reaction: When phenol is treated with an alkyl halide in presence of anhyd. AlCl3, p-cresol is obtained as a major product.
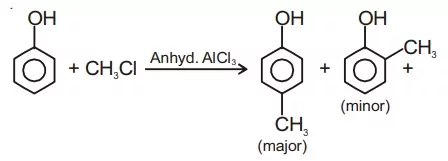

Ans 11. Phenols do not give effervescence (CO2) with NaHCO3 while carboxylic acid reacts.
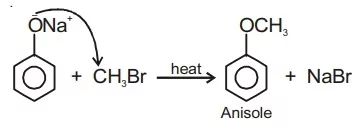
Ans 12. Anisole can be prepared by treating sodium phenoxide with a methyl halide
Ans 14.
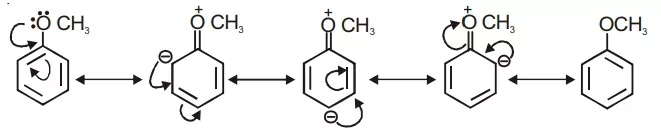
Due to the ‘+R’ effect alternatively, ortho and para positions are becoming negatively charged.
Ans 17. Propan-1-ol < 4-methylphenol < 3-nitrophenol < 3, 5-dinitrophenol < 2, 4, 6-trinitrophenol.
Ans 21. (i) Values associated with this concern is called thinking, problem analysis.
(ii) It can be distinguished by a reaction as given below.
2CH3OH + 2Na → CH3Ona + ½ H2
CH3OCH3 + Na → No Reaction
Methanol reacts with sodium to liberate hydrogen gas and dimethyl ether do not react with sodium.
Ans 22. 1-propoxypropane can be synthesized from propan-1-ol by dehydration.
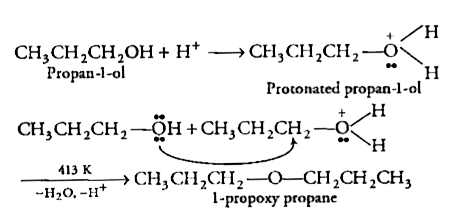
Propan-1-ol undergoes dehydration in the presence of protic acids (such as H2SO4, H3PO4) to give 1-propoxypropane.
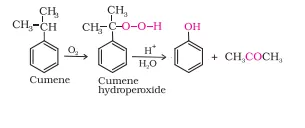
Ans 23. In order To prepare phenol, cumene is first oxidized in the presence of air of cumene hydro-peroxide. Then, cumene hydroxide is treated with dilute acid to prepare phenol and acetone as by-products.
Ans 24. A nitro group is an electron-withdrawing group. … On the other hand, a methoxy group is an electron-releasing group. Thus, it increases the electron density in the O-H bond and hence, the proton cannot be given out easily. For this reason, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Ans 25. The C-O bond in phenols has some double-bond character due to resonance and hence cannot be easily cleaved by nucleophiles.
Practice Questions (Answers) Chapter 11 Class 12 Chemistry
To get questions click Practice Questions Chapter 11 Class 12 Chemistry