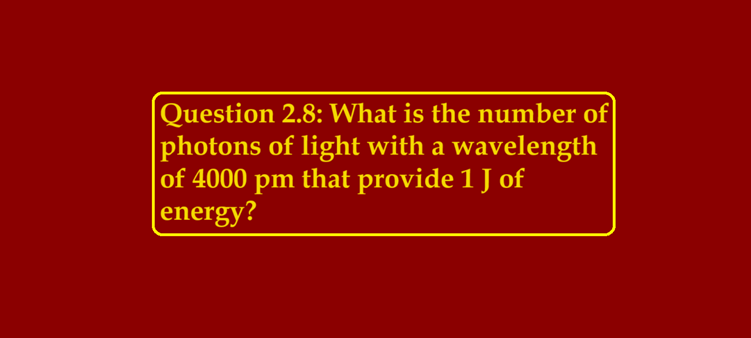Question 2.8: What is the number of photons of light with a wavelength of 4000 pm that provide 1 J of energy?
Question 2.8: What is the number of photons of light with a wavelength of 4000 pm that provide 1 J of energy?
Ans 2.8: Energy (E) of a photon = hν
Energy (En) of ‘n’ photons = nhν
ν = c/Λ
n = EnΛ /hc
Where, λ = wavelength of light = 4000 pm = 4000 ×10–12 m
c = velocity of light in vacuum = 3 × 108 m/s
h = Planck’s constant = 6.626 × 10–34 Js
Substituting the values in the given expression of n:
n = (1) × 4000 ×10–12 m / 6.626 × 10–34 Js × 3 × 108 m/s
n = 2.012 × 1016
Therefore, the number of photons with a wavelength of 4000 pm and energy of 1 J is 2.012 × 1016.
Advertisement