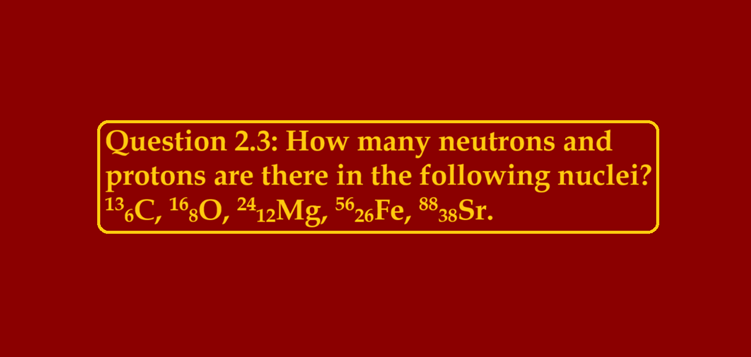Question 2.3: How many neutrons and protons are there in the following nuclei?
136C, 168O, 2412Mg, 5626Fe, 8838Sr
Ans 2.3: 136C
Atomic mass = 13
Atomic number = Number of protons = 6
Number of neutrons = Atomic Mass – Atomic Number
∴ Number of neutrons = 13 – 6 = 7
168O
Atomic mass = 16
Atomic number = 8
Number of protons = 8
Number of neutrons = Atomic Mass – Atomic Number
∴ Number of neutrons = 16 – 8 = 8
2412Mg
Atomic mass = 24
Atomic number = Number of protons = 12
Number of neutrons = Atomic Mass – Atomic Number
∴ Number of neutrons = 24 – 12 = 12
5626Fe
Atomic mass = 56
Atomic number = Number of protons = 26
Number of neutrons = Atomic Mass – Atomic Number
∴ Number of neutrons = 56 – 26 = 30
8838Sr
Atomic mass = 88
Atomic number = Number of protons = 38
Number of neutrons = Atomic Mass – Atomic Number
∴ Number of neutrons = 88 – 38 = 50