Thomsons and Rutherfords Atomic Model
Thomsons and Rutherfords Atomic Model is about the most important atomic models proposed during the study of the structure of atom.
- Types of Atomic Species:
- Isotopes: Atoms of the same element having the same atomic number but a different mass number. For example, isotopes of Hydrogen are 11H, 12H, 13H.
- Isobars: Atoms of different elements having the same mass number but a different atomic number, e.g., 1840Ar, 1940K, 20Ca40
- Isotones: Atoms of different elements which contain the same number of neutrons. e.g., 614C, 715N, 816
- Isoelectronic Species: Atoms or ions containing the same number of electrons. For example, N3-, O2-, F–, Na+, Mg2+, Al3+, and Ne, having 10 electrons each are isoelectronic.
- ATOMIC MODELS:
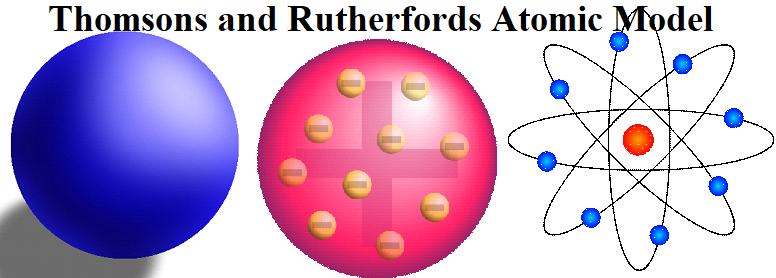
- Thomson’s Model of Atom: J. Thomson proposed the first atomic model, which is known as the plum pudding or raisin pudding, or watermelon model. According to this model, an atom has a spherical shape in which the positive charge is uniformly distributed. The electrons are distributed in it, just like the seeds are distributed in a watermelon or plums are distributed in a pudding. An important feature of this model is that the mass of the atom is assumed to be uniformly distributed over the atom. Also, the total positive charge in an atom is equal to the total negative charge and hence the atom is electrically neutral.
- Rutherford’s Nuclear Model of Atom:
Rutherford proposed an atomic model based on his α–particle scattering experiment. He bombarded a very thin gold foil (approximately 10-7m thickness) with α–particles.
Experiment: A stream of high energy α–particles from a radioactive source was directed at a thin gold foil. The thin gold foil had a circular fluorescent zinc sulphide screen around it. Whenever α–particles struck the screen, a tiny flash of light was produced at that point.

- Observations: The important observations made by Rutherford are:
- Most of the α– particles passed through the gold foil without any
- A small fraction of the α–particles were deflected by small
- A very few α– particles (∼1 in 20,000) bounced back, that is, were deflected by nearly 180°.
- Conclusions: From the above observations, Rutherford made the following conclusions:
- Since most of the α–particles passed through the foil without any deviation, most space in the atom is empty.
- A few positively charged α– particles were deflected. This is because the positive charge of the atom is concentrated in a very small volume at the center called the nucleus.
- The volume occupied by the nucleus is negligibly small as compared to the total volume of the atom. The radius of the atom is about 10–10 m, while that of the nucleus is 10–15
On the basis of the above observations and conclusions, Rutherford proposed the nuclear model (Planetary model) of an atom. According to this model:
- All the positive charge and most of the mass of the atom is concentrated in an extremely small region called the nucleus.
- Electrons are revolving around the nucleus with a very high speed in circular paths called the orbit.
- Electrons and the nucleus are held together by electrostatic forces of attraction.
- Drawbacks of Rutherford Model of an Atom:
- Rutherford’s model cannot explain the stability of the atom.
- He cannot explain the electronic structure of atom.