Properties and Applications of Colloids
Properties of Colloids
(i) Heterogeneous nature: Colloids are heterogeneous in nature and consists of two phases, the dispersed phase and the dispersion medium.
(ii) Visibility: The particles are too small to be seen with the naked eye but become visible when viewed through an ultra-microscope due to the scattering of light by them.
(iii) Surface tension and Viscosity: The surface tension and viscosity of lyophobic sols are not very different from that of the dispersed medium. But, lyophilic sols show higher viscosity and lower surface tension in comparison to the dispersion medium.
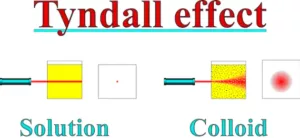
(iv) Tyndall Effect: When a light beam is passed through a colloidal solution, we can see the path of the light beam. This phenomenon is known as the Tyndall effect. It is due to the scattering of a light beam by colloidal particles. The visible path is called the Tyndall cone.
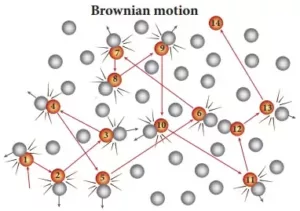
(v) Brownian movement: Zig-zag movement of colloidal particles due to the collision between particles of dispersed phase & dispersion medium, responsible for the stability of colloids.
Properties and Applications of Colloids
Stability of Colloidal Sols: All the dispersed particles in a colloid carry the same charge (either positive or negative) while the dispersion medium has an equal and opposite charge. The particles, therefore, repel one another and do not come close together to form large non-colloidal particles.
The charge on colloidal particles is due to the preferential adsorption of ions, e.g., Fe(OH)3 adsorbs Fe3+ ions from FeCl3 solution and forms positively charged sol. Similarly, AgCl particles can adsorb Cl− ions from chloride solutions or Ag+ ions from solutions having silver ions. The sol will be negatively charged in the first case and positively charged in the second case.
- Negatively charged colloidal sols: Metallic particles like Cu, Au, Pt, Ag etc., starch, clay, silicic acid, metal sulphides like As2S3, CdS, Sb2S3, acidic dyes like congo red, eosin etc.
- Positively charged colloidal sols: Metal hydroxides like Fe(OH)3, Al(OH)3, Cr(OH)3, Ca(OH)2, oxides like TiO2, haemoglobin and basic dyes like methylene blue.
The charge on the sol particles is mainly due to the preferential adsorption of ions from the solution. When 2 or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle takes place. e.g. When AgNO3 is added to KI, AgI is precipitated, which adsorbs iodide ions from the dispersion medium and thus get a negative charge.
But when KI is added to AgNO3, the precipitated AgI adsorbs Ag+ ions from the solution and thus get a positive charge.
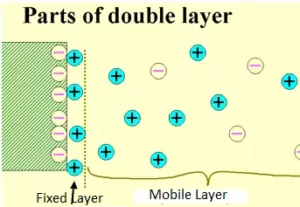
Due to the positive or negative charge in the sol particles, they attract the counterions (opposite ions) from the medium. Thus, a double layer of opposite charges is formed. This is known as a Helmholtz electrical double layer. The layer in which the ions are directly adsorbed to the sol particles is termed as a fixed layer. The second layer is mobile and is termed a diffused layer.
Due to the opposite charges on the fixed and diffused layers, there arises a potential difference between these layers. This potential difference between the fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or zeta potential.
Properties and Applications of Colloids
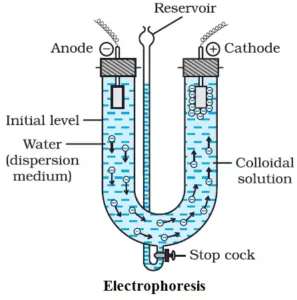
Electrophoresis:
Since colloidal particles carry a charge, they move under the influence of an electric field. This movement of colloidal particles is called electrophoresis. The positively charged sol particles move towards cathode (cataphoresis) and the negatively charged particles move towards the anode (anaphoresis).
Coagulation of colloidal solutions: Process of settling of colloidal particles into precipitate by addition of electrolytes. This can be done in different ways:
- By electrophoresis
- By mixing two oppositely charged sols
- By continuous dialysis
- By boiling
- By the addition of electrolyte
When an electrolyte is added to the sol, the ions carrying the opposite charge to that of the sol neutralize the charge and causes precipitation. The ion of the electrolyte which causes the precipitation is called the coagulating ion or the flocculating ion. A negatively charged ion causes the precipitation of positively charged sol and vice versa
Generally, the greater the valency of the coagulating ion, the greater will be the coagulating power. This is known as Hardy – Schulze rule.
For negatively charged sol, the coagulating power of electrolytes are:
AlCl3 > BaCl2 > NaCl or Al3+> Ba2+> Na+
For positively charged, then the coagulating power of electrolytes follow the following order: K4[Fe(CN)6] > PO43– > SO42– > Cl–.
Coagulating value: The minimum concentration of an electrolyte in millimoles per litre required for the coagulation of a sol within 2 hours is called coagulating value. The smaller the coagulating value, the higher will be the coagulation power.
Protection of colloids: Lyophilic sols are self stabilized, while lyophobic sols require some stabilizing agents. For this purpose, some lyophilic sols are added to lyophobic sols. These lyophilic sols are called protective colloids. When a lyophilic sol is added to a lyophobic sol, the lyophilic particles form a layer around lyophobic particles and thus protect them from electrolytes.
| Difference between Flocculation and Coagulation: When a small amount of the electrolyte is added, i.e., when the concentration of the electrolyte added is low, the process is called flocculation. It can be reversed on shaking. However, at higher concentration, coagulation takes place and the process cannot be reversed simply by shaking. |
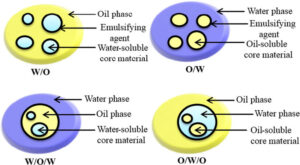
Emulsions: It is a colloidal dispersion in which both the dispersed phase and the dispersion medium are liquids (which are otherwise immiscible). Substances like soaps which help in making the emulsions stable are called emulsifiers or emulsifying agents.
Types of Emulsions: Emulsions are of two types:
1. Oil in water (o/w): Emulsions in which oil is the dispersed phase and water is the dispersion medium. For example, milk and vanishing cream.
2. Water in oil (w/o): Emulsions in which water is the dispersed phase and oil is the dispersion medium. For example, cod liver oil, butter and cream.
Dye test to distinguish between Emulsions:
Demulsification: The process of breaking an emulsion to yield constituent.
Properties and Applications of Colloids
Properties and Applications of Colloids
Applications of Colloids:
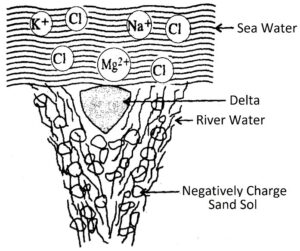
(1) Formation of Delta: Deltas are formed at the river mouth. This is because the river water is a negatively charged colloid of sand particles. When this water enters into the sea, the positive ions present in seawater coagulate the colloidal solution of sand and so the particles settle down. This will result in the formation of the delta.
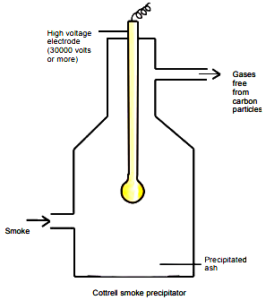
(2) Electrical precipitation of smoke (Cottrell precipitation): Smoke is a colloidal solution of carbon, arsenic compounds, dust particles etc. in the air. The smoke before coming out of the chimney is passed through a chamber (Cottrell precipitator) containing plates having a charge opposite to that of smoke particles. Thus, neutralization of charges occurs and the particles settle down and pure air flows out of the chimney.
(3) Purification of drinking water: The water obtained from natural sources often contains suspended impurities. In order to coagulate these impurities, alum is added to water. The positive ions present in alum neutralize the suspended impurities and hence get purified.
(4) Medicines: Most of the medicines are colloidal in nature. This is because they have a large surface area and are therefore easily assimilated. For example, Argyrol is a silver sol used as an eye lotion. Colloidal antimony is used in curing kala-azar (Visceral disease, the most devastating and fatal form of leishmaniasis, is classically known as kala-azar or the Indian name for “black fever/disease). Colloidal gold is used for intramuscular injection.
(5) Tanning: Animal hides are colloidal in nature. When a hide, which has positively charged particles, is soaked in tannin (which contains negatively charged colloidal particles) mutual coagulation takes place. This results in the hardening of leather. This process is termed as tanning.
(6) Photographic plates and films: Photographic plates or films are prepared by coating an emulsion of the light-sensitive silver bromide in gelatin over glass plates or celluloid films.
(7) Rubber industry: Rubber latex is a colloidal solution of rubber particles that are negatively charged. Rubber is obtained by coagulation of the latex.
(8) Food articles: Milk, butter, halwa, ice creams, fruit juices, etc., are all colloids in nature.
(9) Blood: Blood is a colloidal solution of an albuminoid substance. When alum and ferric chloride (FeCl3) solution are added to blood, then coagulation of particles takes place which results in the clotting of blood.
Properties and Applications of Colloids