Isomerism in Coordination Compounds Class 12
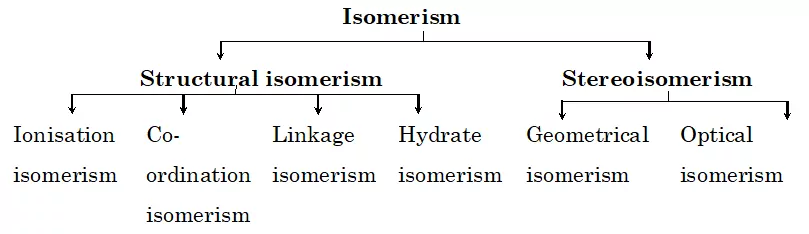
Isomerism
Two or more chemical compounds which have identical chemical formula but different structures are known as isomers and the phenomenon is known as isomerism. The isomers have different arrangements of ligands around the central metal atom. The isomerism shown by co-ordination compounds are broadly divided into two:
Structural Isomerism and Stereo Isomerism.
Structural Isomerism: These are isomers that differ in the structural arrangement of ligands around the central atom. They are of four types:
(a) Ionisation Isomerism
(b) Coordinate Isomerism
(c) Linkage Isomerism
(d) Hydrate Isomerism
Isomerism in Coordination Compounds Class 12
(a) Ionisation Isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion. Example: [Co(NH3)5Br]SO4 and [Co(NH3)5 SO4]Br,
[Pt(OH)2(NH3)4]SO4 and [Pt (SO4)(NH3)4](OH)2,
[Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl2.
(b) Coordinate Isomerism: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
Example: [Co(NH3)6] [Cr(C2O4)3] and [Cr(NH3)6] [Co(C2O4)3],
[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
[Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6]
(c) Linkage Isomerism: This isomerism takes place when a monodentate ligand has two possible donor atoms and attaches in two different ways to the central metal atom. Such ligands are known as also ambidentate ligands. For example, nitro (NO2– ) and nitrito (-ONO–), cyano (-CN–), and isocyano (-NC–).
For Example: [Cr(H2O)5SCN]2+ and Cr(H2O)5NCS]2+, [Co(NO2)(NH3)5]Cl2 and [Co(ONO)(NH3)5]Cl2.
(d) Hydrate Isomerism: It is isomerism in which solvent is involved as ligand. If the solvent is water it is called hydrate isomerism. This form of isomerism is also known as ‘solvate isomerism’.
For Example: [Cr(H2O)6]Cl3 (violet) and its solvate isomer [Cr(H2O)5Cl]Cl2.H2O (grey-green).
Isomerism in Coordination Compounds Class 12
Stereo-isomerism: In stereo-isomerism, the isomers differ only in the spatial arrangement of atoms or groups about the central metal atom. It is also known as space isomerism. It can be further classified into two types:
(i) Geometrical isomerism (ii) Optical isomerism
(i) Geometrical isomerism: This isomerism is due to the difference in the geometrical arrangement of the ligands around the central atom. . It is mainly found in co-ordination complexes with co-ordination numbers 4 (square planar complexes) and 6 (octahedral complexes). When similar ligands are on adjacent positions, it is known as cis form and when these are in the opposite positions, it is known as trans-form. Thus, this is also known as cis-trans isomerism.
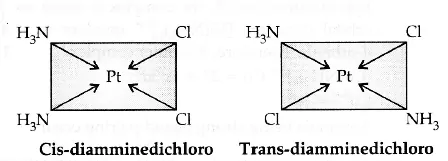
(a) Square planar complex of formula [MX2L2] (X and L are unidentate): The two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer. e.g., [Pt(NH3)2Cl2].
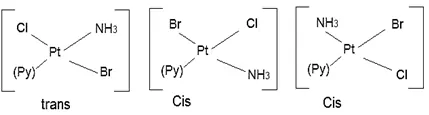
(b) Square planar complex of the type [MABXL] (where A, B, X, L are unidentates): Shows three isomers– two cis and one trans. Such isomerism is not possible for tetrahedral geometry. e.g., [Pt(NH3)(Br)(Cl)(Py)].
(c) Octahedral complexes of formula [MX2L4]: In which the two ligands X may be oriented cis or trans to each other. e.g., [Co(NH3)4Cl2]+
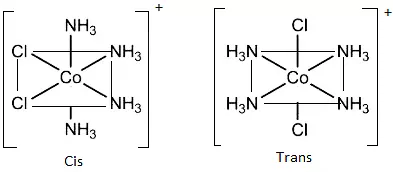
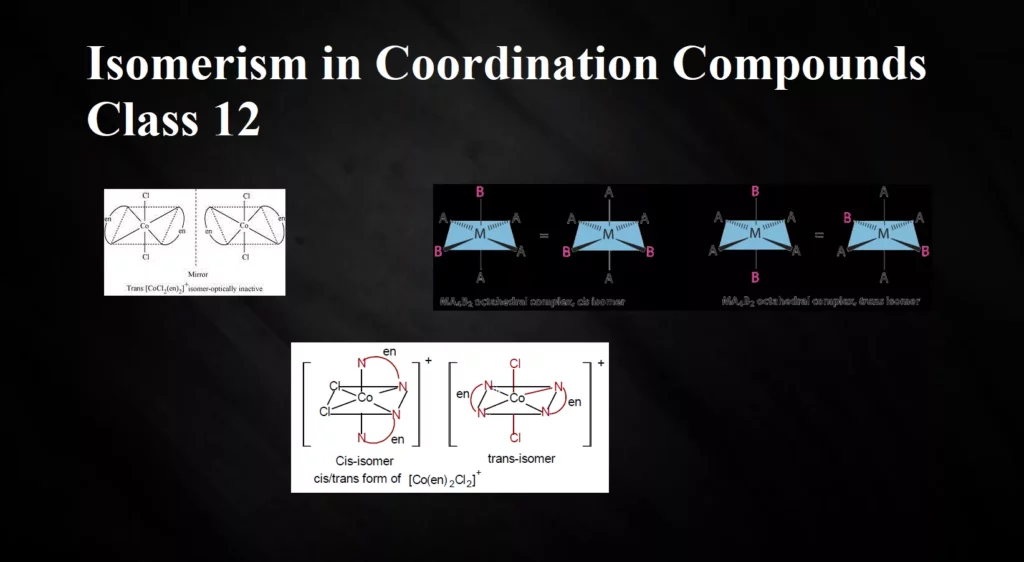
(d) Octahedral complexes of formula [MX2A2]: Where X is unidentates and A are bidentate and form cis and trans isomers. e.g., [CoCl2(en)2].

(e) Octahedral coordination entities of the type [MA3B3] Like [Co(NH3)3(NO2)3]: If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac) isomer. When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer.
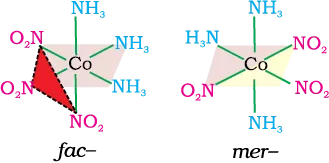
Isomerism in Coordination Compounds Class 12
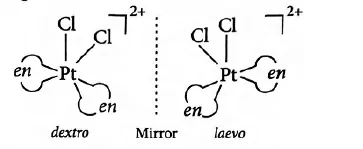
(ii) Optical Isomerism: Optical isomers are mirror images that cannot be superimposed on one another. These are also called enantiomers. The molecules or ions that cannot be superimposed are called chiral. There are two forms of optical isomers – Dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right, l to the left). Optical isomerism is common in octahedral complexes involving bidentate ligands. In a co-ordination entity of the type [PtCl2(en)2]+, only the cis-isomer shows optical activity. The trans-isomer has a plane of symmetry and is optically inactive.
[M(AA)3] type, [M(AA)2B2] or [M(AA)2BC] type and [M(AA)B2C2] type
Square planar complexes: Generally square planar complexes are not optically active as they have all the ligands and metal atoms in one plane. That is why there is a plane of symmetry.
Isomerism in Coordination Compounds Class 12