Electrolysis of NaCl When Pt Electrode
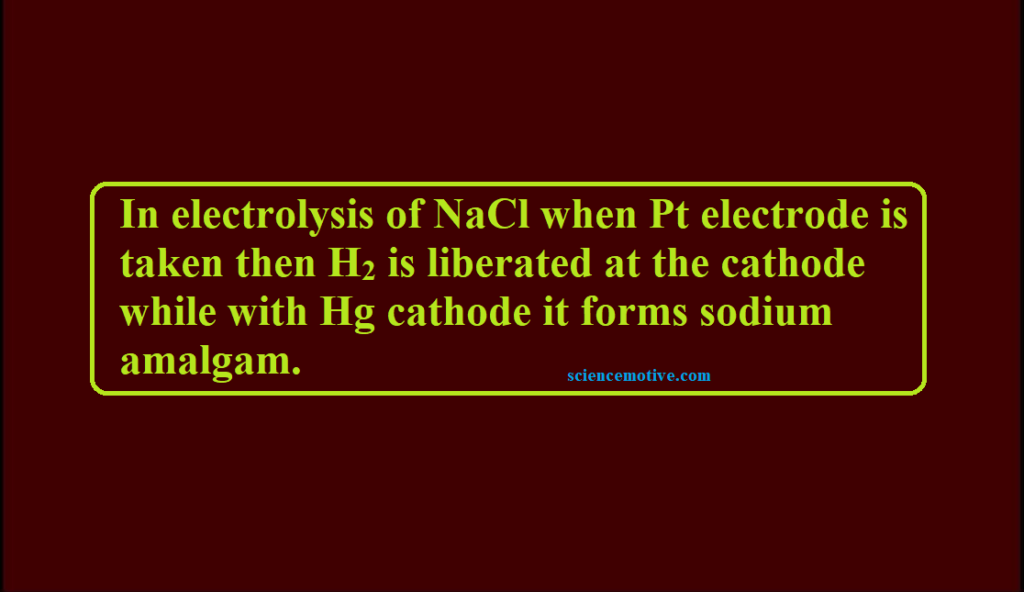
Que. In electrolysis of NaCl when Pt electrode is taken then H2 is liberated at the cathode while with Hg cathode it forms sodium amalgam. The reason for this is
(a) Hg is more inert than Pt
(b) more voltage is required to reduce H+ at Hg than at Pt
(c) Na is dissolved in Hg while it does not dissolve in Pt
(d) conc. of H+ ions is larger when Pt electrode is taken.
Ans. (b)
When sodium chloride is dissolved in water, it ionizes as NaCl ![]() Na+ + Cl–.
Na+ + Cl–.
Water also dissociates as H2O ![]() H+ + OH–
H+ + OH–
During the passing of electric current through this solution using a platinum electrode, Na+ and H+ ions move towards the cathode. However, only H+ ions are discharged more readily than Na+ ions because of their low discharge potential (in the electromotive series hydrogen is lower than sodium). These H+ ions gain electrons and change into a neutral atom
At cathode H+ + e– → H, H + H → H2
Cl– and OH– ions move towards the anode. Cl– ions lose electrons and change into neutral atoms.
At the anode, Cl– – e– → Cl, Cl + Cl → Cl2
If mercury is used as a cathode, H+ ions are not discharged at the mercury cathode because mercury has a high hydrogen overvoltage. Na+ ions are discharged at the cathode in preference to H+ ions, yielding sodium, which dissolves in mercury to form sodium amalgam.
At cathode: Na+ + e– → Na
Electrolysis of NaCl When Pt Electrode