d and f block elements Notes
Introduction
The elements lying in the middle of the periodic table belonging to groups 3 to 12 are known as d – block elements.
The elements in which the last electron enters into the d-orbitals of the penultimate shell i.e. (n–1) d where n is the last shell are called d-block elements.
They are placed in between s-block and p-block elements.
They show a regular transition from the highly electropositive metals of s-block elements to the less electropositive p-block elements. So, they are called transition elements.
Transition elements
A transition element is defined as the one which has incompletely filled d orbitals in its ground state or in any one of its oxidation states.
Zinc, cadmium, mercury are not regarded as transition metals due to completely filled d – orbitals in both atomic and ionic state.
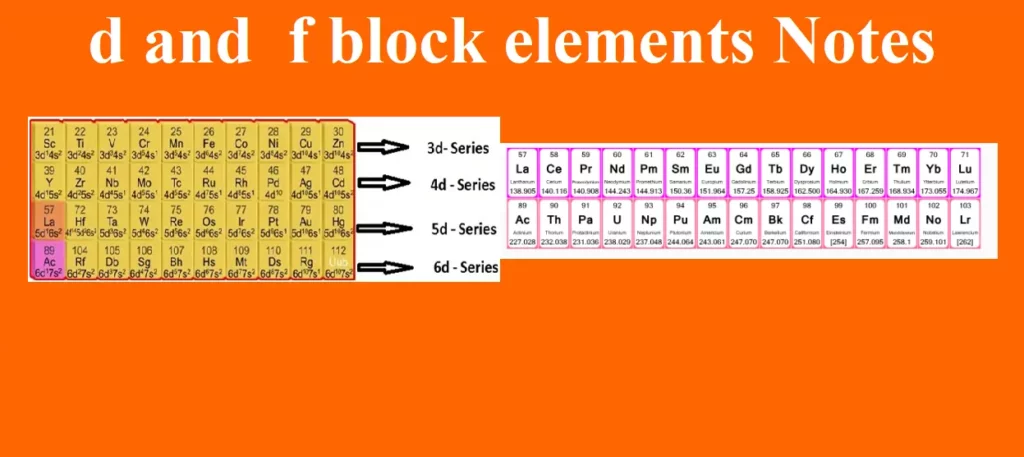
The four series of transition element are:-
First transition series → Sc21 to Zn30
Second transition series → Y39 to Cd48
Third transition series → La57, Hf72 to Hg80
Fourth transition series → Ac89, Rf104 onwards (incomplete)
Electronic configuration: General outer electronic configuration of d-block elements is (n-1) d1-10 ns1-2. There is only a small difference in energy between (n-1)d orbital and ns orbital. So in some cases, ns electrons are also transferred to (n-1)d level. The electronic configurations of Cr and Cu in the 3d series show some exceptions.
24Cr – [Ar] 3d5 4s1
29Cu – [Ar] 3d10 4s1
This is due to the extra stability of half-filled and completely-filled electronic configurations. (d5 or d10).
d and f block elements Notes
General Characteristics of transition element:
(1) Atomic Radii: The atomic radii decrease from Sc to Cr because the effective nuclear charge increases. The atomic size of Fe, Co, Ni is almost the same because the attraction due to the increase in nuclear charge is cancelled by the repulsion because of an increase in shielding effect. Cu and Zn have a bigger size because of the shielding effect increases and electron-electron repulsions repulsion increases. 
(2) Ionic Radii: The atomic and ionic radii of 2nd and 3rd row transition metals are quite similar. This is due to the Lanthanoid contraction. In between the 2nd and 3rd row transition elements, 4f electrons are present. The 4f electrons have a very poor shielding effect and as a result the atomic and ionic radii of Lanthanoid decrease from left to right (Lanthanoid contraction).
(3) Melting and Boiling Points: The transition metals have very high m.p. and b.p. The high melting points of these metals are attributed to the involvement of a greater number of the electron from (n-1)d in addition to the ns electrons. This can also be explained in terms of metallic bond strength which depends on the number of unpaired electrons. As the number of unpaired electron increases, the metallic bond strength increases. Hence the melting point also increases. In a given transition series, the number of unpaired electrons increases up to the middle and then decreases. Another factor that affects the m.p is the heat of atomization. Mn and Tc have a low melting point even though they have d5 configuration. This is because of their low heat of atomization. 
(4) Ionisation Enthalpy: There is slight and irregular variation in ionization energies of transition metals due to irregular variation of atomic size. The I.E. of the 5d transition series is higher than the 3d and 4d transition series because of Lanthanoid Contraction. 
| Lanthanoid Contraction: The steady decrease in the atomic and ionic radii of the transition metals as the atomic number increases. This is because of the filling of 4f orbitals before the 5d orbitals. This contraction in size is quite regular. This is called the lanthanoid contraction. It is because of lanthanoid contraction that the atomic radii of the second row of transition elements are almost similar to those of the third row of transition elements. |
(5) Metallic Character: All transition elements are metallic in nature, i.e. they have strong metallic bonds. This is because of the presence of unpaired electrons. This gives rise to properties like high density, high enthalpies of atomization, and high melting and boiling points.
d and f block elements Notes
(6) Oxidation State: Transition metals show variable oxidation states. This is because in these elements d and s electrons have comparable energies. So, in chemical reaction along with s-electrons, d-electrons also participate. In a given transition series, the maximum oxidation state increases up to the middle and then decreases.
The most common oxidation states are in circles
(7) Electrode Potential: The electrode potential values of first-row transition series generally increases from left to right with some exceptions. The E0(Cu2+/Cu) is positive (+0.34V), while the E0 values of all the other first-row transition elements are –ve. This is because the high energy required to transform Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy. So, Cu does not easily react with acid and liberate H2. Only oxidizing acids [HNO3 and hot Conc. H2SO4] react with Cu and the acid get reduced. 
(8) Magnetic properties Paramagnetic: Due to the presence of the unpaired electrons in (n-1)d orbitals, most of the transition metal ions and their compounds are paramagnetic. They are attracted by the magnetic field. Diamagnetic – They have paired electrons and are repelled by a magnetic field.
Magnetic moment µs = √n(n+2), n = number of unpaired electrons Magnetic moment or paramagnetic property increases with an increase in the number of unpaired electrons. Ferromagnetic – Substance which is attracted very strongly is said to be ferromagnetic. Ferromagnetism is an extreme form of paramagnetism.
Enthalpy of atomisation, ΔaH0, is the change in enthalpy when one mole of bonds are completely broken to obtain atoms in the gas phase.
(9) Enthalpy of Atomization: Transition elements (except Zn, Cd and Hg) are much harder and less volatile. They exhibit high enthalpies of atomization. The maximum value lies at about middle of each series indicating the interatomic interaction increases with the number of unpaired d-electrons. The metals of 4d and 5d transition series have greater enthalpies of atomization than the corresponding elements of the first (3d) series. This is due to much more frequent metal-metal bonding in their compounds. 
(10) Formation of Coloured Compounds: They form coloured ions due to the presence of partially filled d – orbitals and unpaired electrons, they can undergo d – d When an electron from a lower energy d orbital is excited to a higher d level, it absorbs energy and this is equal to the energy of certain colours in the visible region. So, the colour observed is the complementary colour of the light absorbed. 
(11) Formation of complexes: Transition metals form complexes due to, Presence of vacant d – orbitals of suitable energy, Smaller size, Higher charge on cations, Ability to show a variable oxidation state. Eg: K4[Fe(CN)6], K3[Fe(CN)6], [Ni(CO)4] etc.
d and f block elements Notes
(12) Catalytic Properties: Most of the transition metals are used as catalyst because of the presence of incomplete or empty d – orbitals, Large surface area, Variable oxidation state, Ability to form complexes E.g., Fe, Ni, V2O3, Pt, Mo, Co and used as a catalyst.
(13) Interstitial Compounds: Transition metals have empty spaces or interstitial sites in which non-metals C, H, N, B etc. can fit into resulting in the formation of interstitial compounds. They are non – stoichiometric, i.e., their composition is not fixed, e.g., steel. They are harder and less malleable and ductile E.g.: Fe3H, Mn4N, TiC, VH56, TiH1.7 etc. Some of the properties of these compounds are: They have a high melting point, They are very hard, They retain metallic conductivity, They are chemically inert. 
(14) Alloy Formation: Alloys are homogeneous solid solutions of elements in which at least one element is a metal. They are formed by atoms with metallic radii within about 15% of each other. Because of similar radii and other characteristics of Transition metals, they readily form alloys. The alloys formed are hard and have high m.p. e.g.: Bronze (Cu, Zn), Stainless steel (Fe, C, Ni, Mn and Cr). 
d and f block elements Notes
Preparation and Properties of Potassium Permanganate and Potassium Dichromate
Preparation and Properties of Potassium Permanganate and Potassium Dichromate:
General Properties of First Row Transition Metal Compounds Oxides and oxometal ions:
(i) Oxides of metals in low oxidation states + 2 and + 3 (MO, M3O4 and M2O3) are generally basic except Cr2O3 which is amphoteric in character.
(ii) Oxides of metals in higher oxidation states + 5 (M2O5, MO3, M2O7) are generally acidic in character.
(iii) Oxides of metals in their intermediate oxidation states + 4 (MO2) are generally amphoteric in nature. Besides the oxides, the oxocations, which stabilise V (V) species is VO2+, V (IV) species is VO2+ and Ti(IV) species is TiO2+.
(iv) As the oxidation number of the metal in the oxide increases, ionic character decreases and acidic character increases.
Thus, +2 +8/3 +3 +4 +7
MnO Mn3O4 Mn2O3 MnO2 Mn2O7
→ Ionic Character Decreases
→ Acidic Character Increases
Preparation of Potassium dichromate (K2Cr2O7): It is prepared by the reaction of chromate ore (FeCr2O4) with sodium carbonate in excess of air.
Step 1: Conversion of chromite ore to sodium chromate
4 FeCr2O4 + 8 Na2CO3 + 7 O2 → 8 Na2CrO4 + 2 Fe2O3 + 8 CO2
Step 2: Acidification of sodium chromate to sodium dichromate
2Na2CrO4 + 2 H+ → Na2Cr2O7 + 2 Na+ + H2O
Step 3: Conversion of sodium dichromate to potassium dichromate
Na2Cr2O7 + 2 KCl → K2Cr2O7 + 2 NaCl
Physical properties:
It forms orange-red crystals which melt at 396 0
It is moderately soluble in cold water but freely soluble in hot water.
Chemical properties:
The chromate and dichromate are inter-convertible in an aqueous solution depending upon pH of the solution. Chromate on acidification gives dichromate and the dichromate on treating with alkali gives chromate.
2 CrO42– + 2H+ → Cr2O72– + H2O
Cr2O72– + 2OH– → 2 CrO42– + H2O
The oxidation state of chromium in chromate and dichromate is +6.
The structures of chromate ion, CrO42– and the dichromate ion, Cr2O72– are shown below.
Sodium and potassium dichromates are strong oxidising agents. The sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry.
The action of Alkalies: When an alkali is added to an orange-red solution of dichromate, a yellow solution results due to the formation of chromate.
K2Cr2O7 + KOH → K2CrO4 + H2O
Oxidising Properties: K2Cr2O7 is a good oxidising agent in an acidic medium. Its oxidising action can be represented as follows:
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O
Thus, acidified potassium dichromate will oxidise
d and f block elements Notes
(i) Iodides to iodine
6 I – → 3I2 + 6 e–
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O
—————————————————–
6I – + Cr2O72– + 14H+ → 3I2 + 2Cr3+ + 7H2O
(ii) Sulphides to sulphur
3S2- →3 S + 6e–
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O
—————————————————–
3S2- + Cr2O72– + 14H+ → 3S + 2Cr3+ + 7H2O
————————————————-
(iii) Tin(II) to tin(IV)
3 Sn2+ → 3Sn4+ + 6 e–
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O
———————————————–
3Sn2+ + Cr2O72– + 14H+ → 3 Sn4+ + 2Cr3+ + 7H2O
————————————————————-
(iv) Iron(II) (ferrous) to iron(III) (ferric)
6 Fe2+ → 6Fe3+ + 6 e–
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O
—————————————————–
Cr2O72– + 14 H+ + 6 Fe2+ → 2 Cr3+ + 6 Fe3+ + 7 H2O
—————————————————————-
(v) It oxidises ethyl alcohol to acetaldehyde and acetic acid
K2Cr2O7 + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O +3O
CH2CH2OH + O → CH3CHO + H2O
CH3CHO + O → CH3COOH
Acetaldehyde Acetic Acid
Test for a drunken driver. The above reaction helps to test whether a driver has consumed alcohol or not. he is asked to breathe into the acidified K2Cr2O7 solution taken in a test tube. If the orange colour of the solution changes into green colour (due to Cr2(SO4)3 formed in the reaction), the driver is drunk, otherwise not.
Chromyl chloride test (Reaction with chloride and conc. sulphuric acid): When heated with concentrated hydrochloric acid or with chloride and strong sulphuric acid, reddish-brown vapours of Chromyl Chloride are obtained.
K2Cr2O7 + 6 H2SO4 + 4 KCl → CrO2Cl2 + 6 KHSO4 + 3H2O
Chromyl Chloride
Uses:
(i) In volumetric analysis, it is used as a primary standard for the estimation of Fe2+ (ferrous ions) and I– (iodides) in redox titrations.
(ii) In industry, it is used
(a) In chrome tanning in the leather industry.
(b) In the preparation of chrome alum K2SO4.Cr2(SO4)3. 24H2O and other industrially important compounds such as Cr2O3, CrO3, CrO2Cl2, K2CrO4, CrCl3 etc.
(c) In calico printing and dyeing.
(d) In photography and in hardening gelatine film.
d and f block elements Notes
Preparation of Potassium Permanganate (KMnO4): Potassium permanganate is commercially prepared from Pyrolusite (MnO2). The preparation involves two steps.
Step 1: MnO2 is fused with KOH to form potassium manganate (K2MnO4).
2 MnO2 + 4 KOH + O2 → 2 K2MnO4 + 2 H2O
Step 2: K2MnO4 is electrolytically oxidised to potassium permanganate.
3 MnO42- + 4 H+ → 2 MnO4– + MnO2 + 2 H2O
Properties: Potassium permanganate forms dark purple crystals which are iso-structural with those of KClO4. When heated it decomposes and liberate O2.
2KMnO4 → K2MnO4 + MnO2 + O2
The manganate and permanganate ions are tetrahedral

The green manganate is paramagnetic with one unpaired electron but the permanganate is diamagnetic.
(i) Colour: Potassium permanganate exists as a deep purple-black colour with a greenish lustre which becomes dull in the air due to superficial reduction.
(ii) Solubility: It is moderately soluble in water at room temperature and it is more soluble in hot water.
(iii) Action of heat: When heated to 513 K, it readily decomposes giving oxygen.
KMnO4 → K2MnO4 + MnO2 + O2
At red heat, potassium manganate formed decomposes into potassium manganite (K2MnO3) and oxygen
K2MnO4 → K2MnO3 + O2
The action of heat in a current of Hydrogen: When heated in a current of H2, solid KMnO4 gives KOH, MnO and water vapours.
2 KMnO4 + 5 H2 → 2 KOH + 2 MnO2 + 4 H2O
Oxidising properties:
KMnO4 is a good oxidizing agent in acidic, basic and neutral media. The oxidizing action in acidic medium is due to the reaction:
MnO4– + 8H++ 5e–→ Mn2+ + 4H2O
Acidified permanganate solution oxidises:
(i) Oxalates to carbon dioxide
5 × (C2O42-→2 CO2 + 2e–)
2 × (MnO4– + 8H++ 5e–→ Mn2+ + 4H2O)
5 C2O42- +2 MnO4– + 16H+ → 10 CO2 +2 Mn2+ + 8H2O
——————————————————————
(ii) Iron(II) to iron(III)
5 Fe2+ → 5 Fe3+ + 5e–
MnO4– + 8H++ 5e–→ Mn2+ + 4H2O
5Fe2+ + MnO4– + 8H+ → 5Fe3+ + Mn2+ + 4H2O
——————————————————-
(iii) Nitrites to nitrates
5NO2– + 5H2O → 5NO3– + 10H+ + l0e–
2 × (MnO4– + 8H++ 5e–→ Mn2+ + 4H2O)
—————————————————————
5NO2– +2 MnO4– + 6H+ → 5NO3– + 2 Mn2+ + 3H2O
————————————————————
In alkaline or neutral medium, permanganate ion is reduced to
MnO2 MnO4– + 2H2O + 3e–→ MnO2 + 4OH–
In alkaline medium it oxidises:
(i) Iodide to iodate
(MnO4– + 2H2O + 3e–→ MnO2 + 4OH–) × 2
I– + 6OH– → IO3– + 3 H2O + 6 e–
——————————————————
MnO4– + H2O + I–→ 2MnO2 + 2OH–+ IO3
——————————————————
(ii) Thiosulphate to sulphate
(MnO4– + 2H2O + 3e–→ MnO2 + 4OH–) × 8
(S2O32- + 10OH– → 2SO42- + 5 H2O + 8e-) × 3
—————————————————————-
8MnO4– + H2O +3 S2O32- → 8MnO2 + 2OH–+ 6SO42-
—————————————————————-
Uses: It is used as an oxidising agent in acidic, basic and neutral medium. It is used as a primary standard in volumetric analysis. It is used for the bleaching of wool, cotton, silk and other textile fibres and also for the decolourisation of oils.
d and f block elements Notes
f-Block Elements and Properties
The elements in which the last electron enters the anti-penultimate f-subshell are called f-block elements. They include lanthanides of the 6th period and actinides of the 7th period.

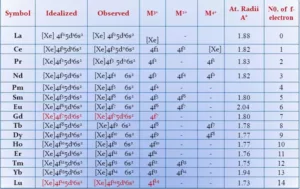
The Lanthanoids: Elements’ filling up of 4f – orbitals are called Lanthanoids series. The 14 elements after lanthanum of the 6th period are called lanthanides or lanthanoids or lanthanones or rare-earth. They include elements from 58Ce to 71Lu. They are generally represented as Ln.
(i) Atomic and Ionic Radii: The overall decrease in atomic and ionic radii from Lanthanum to Lutetium is a unique feature in the chemistry of the lanthanoids. This regular decrease is known as lanthanoid contraction. In lanthanides, as the atomic number increases, the nuclear charge increases one by one and the electrons are added to the anti-penultimate f subshell. Due to its diffused shape, f orbitals have a poor shielding effect. So the nucleus can attract the outermost electrons strongly and as a result, the radii decreases.
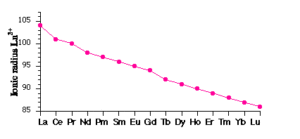
Consequences of Lanthanoid Contraction:
a) It results in a slight variation in their chemical properties which helps in their separation by ion-exchange methods.
b) Each element beyond lanthanum has the same atomic radius as that of the element lying above it in the same group (e.g., Zr 145 pm, Hf 144 pm); Nb 134 pm, Ta 134 pm; Mo 129 pm, W 130 pm).
c) The covalent character of hydroxides of lanthanoids increases as the size decreases from La3+ to Lu3+. Hence, the basic strength decreases. Thus, La(OH)3 is the most basic whereas Lu(OH)3 is the least basic. Similarly, the basicity of oxides also decreases in the order from La3+ to Lu3+.
d) Tendency to form stable complexes from La3+ to Lu3+ increases as the size decreases in that order.
(ii) Oxidation State: In lanthanoids, the most common oxidation state is +3. However, +2 and +4 ions in solution or in solid compounds are also obtained. This irregularity arises mainly from the extra stability of empty, half-filled or filled f subshells. Cerium shows the oxidation state +4 due to its noble gas configuration. Pr, Nd, Tb and Dy also exhibit +4 state but only in oxides, MO2. Eu and Yb show +2 oxidation state because of the stable f7 or f14 configuration. Sm shows +2 oxidation state also.
(iii) Colour: Most of the trivalent lanthanoid ions are coloured both in the solid-state and in an aqueous solution. This is due to the partly filled f-orbitals which permit f-transition.
La3+ (colourless) Lu3+ (colourless)
Ce3+ (colourless) Yb3+ (colourless)
Pr3+ (yellow green) Tm3+ (green)
Nd3+ (red) Er3+ (pink)
Pm3+ (uncertain) Ho3+ (Yellow)
Sm3+ (yellow) Dy3+ (yellow)
Eu3+ (pink) Tb3+ (pink)
Gd3+ (pink)
(iv) Complex Formation: Although the lanthanoid ions have a high charge (+3) yet the size of their ions is very large yielding a small charge to size ratio, i.e., low charge density. As a consequence, they have a poor tendency to form complexes.
(v) Reducing Character: The E° values for M3+; M3+(aq) + 3e− → M(s) lie in the range of – 2.2 to -2.4 V (the exception being Eu, E° = – 2.0V) indicating thereby that they are highly electropositive, readily lose electrons and thus are good reducing agents.
d and f block elements Notes
Chemical Behaviour:
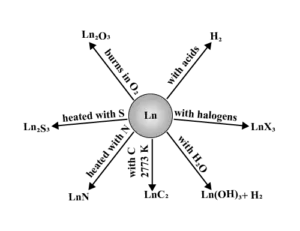

The Actinoids: The elements with atomic numbers 90 to 103, i.e., thorium to lawrencium (which come immediately after actinium, Z = 89 are called actinoids or actinides or actinones. These elements involve the filling of 5f-orbitals and are also called 5f-block elements or second inner transition series. Their general electronic configuration is [Rn] 5f1−14 6d0−1 7s2.
Most of them are artificially prepared and are short-lived. They are radioactive. The elements after Uranium are artificially prepared and so they are called trans-uranium elements or trans-uranic elements.
(i) Atomic and Ionic Radii: In the actinoid series, the atomic and ionic radii decrease regularly from left to right. This is known as Actinoid contraction.
(ii) Oxidation States: The exhibition of a large number of oxidation states of actinoids is due to the fact that there is a very small energy gap between 5f, 6d and 7s subshells and thus all their electrons can take part in bond formation.
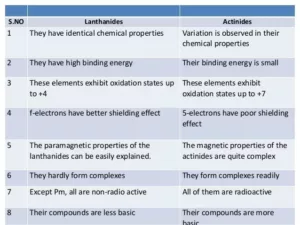
Comparison between Lanthanoids and Actinoids
Uses of Actinoids:

d and f block elements Notes
f-Block Elements and Properties
https://sciencemotive.com/class-12-chemistry/f-block-elements-and-properties/