Coordination Compounds Class 12 Notes
Important Topics
The concept of coordination compounds originates from the tendency for the complex formation of the transition elements.
Complex compounds or coordination compounds are those molecular compounds that retain their identity in solid and in solution are known as complex compounds.
K4[Fe(CN)6 ] + H2O → 4K+(aq) + [Fe(CN)6]4− (aq)
Molecular or Addition Compounds:
When solutions of two or more simple stable compounds in molecular proportion are allowed to evaporate, crystals of new substances, called molecular or addition compounds, are obtained. For Example:
CuSO4 + 4NH3 → CuSO4·4NH3
AgCN + KCN → KCN·AgCN
There are two types of molecular or addition compounds: Double salts or lattice compounds and Coordination or complex compounds.
(1) Double salts or lattice compounds: These are the molecular compounds that exist only in the solid-state and lose their identity when dissolved in water, i.e., they dissociate into simple ions completely when dissolved in water. For Example FeSO4. (NH4)2SO4.6H2O (Mohr’s Salt), K2SO4. Al2(SO4)3.24H2O (Potash Alum).
(2) Coordination or Complex Compounds: These are the molecular compounds that retain their identity in the solid as well as in the dissolved state and their properties are completely different from those of their constituent particles. A part of these compounds is not dissociated in solution and its behavior is different from its constituents.
For example, potassium argentocyanide does not form Ag+ and CN– simpler ions but instead, gives argentocyanide complex ion [Ag(CN)2]– (which does not dissociate).
Cationic complexes: A complex in which the complexion carries a net positive charge is called a cationic complex. For Example, [CoCl2(en)2]+, [Co(NH3)6]3+, [Cu(NH3)4]2+.
Anionic complexes: A complex in which the complexion carries a net negative charge is called anionic complex, e.g., [Ag(CN)2]–, [Fe(CN)6]4-, [Fe(C2O4)3]3−.
Neutral complexes: A complex carrying no net charge is called a neutral complex or simply a complex e.g., [Ni(CO)4], [Co(NH3)3Cl3], [Ni(CO)4].
Coordination Compounds Class 12 Notes
Some Important Definition
1) Coordination Entity: Central atom/ion (metal) to which a fixed number of other atoms or groups are attached (ligand). For example, [CoCl3(NH3)3] is a coordination entity in which the cobalt ion is surrounded by three ammonia molecules and three chloride ions. Other examples are [Ni(CO)4], [PtCl2(NH3)2], [Fe(CN)6]4–, [Co(NH3)6]3+.
A coordination entity can be neutral or charged.
2) Central atom/ion: In a coordination entity, the atom/ion to which a fixed number of ions/neutral molecules are attached is called the central atom or ion. For example, the central atom/ion in the co-ordination entities: [NiCl2(H2O)4], [CoCl(NH3)5]2+ and [Fe(CN)6]3– are Ni2+, Co3+ and Fe3+ respectively.
These central atoms/ions are also referred to as Lewis acids since they accept electron pairs from ligands.
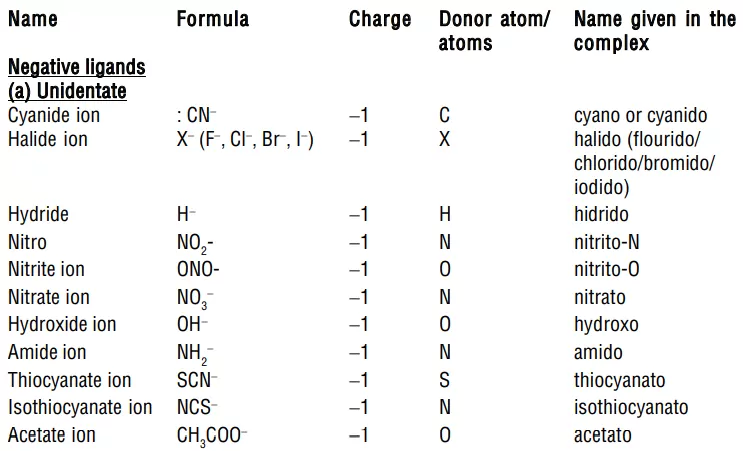
3) Ligands: The anions, cations, or neutral molecules, which form coordinate bonds with the central metal atom by donating an electron pair (lone pair) are ligands. These electron-pair donors are also known as Lewis bases. The atom in the ligand which donates the electron pair is called the donor atom or ligating group. The ligands containing one, two, or more donor atoms are known as unidentate, bidentate, or multidentate respectively. For Example, Examples for ligands are Cl−, Br−, F−, I−, OH−, CN−, NC−, CNO−, NCO−, SO42−, NO3−, CNS–, H2O, NH3, CO, etc.
Classification of Ligands:
Ligands are classified as follows:
(a) On the basis of the charge of ligand:
i) Anionic ligands: These are negatively charged and are the most common type of ligand, such as F–, Cl–, Br–, OH–, CN–, SO32–, S2–, SO42–, etc.
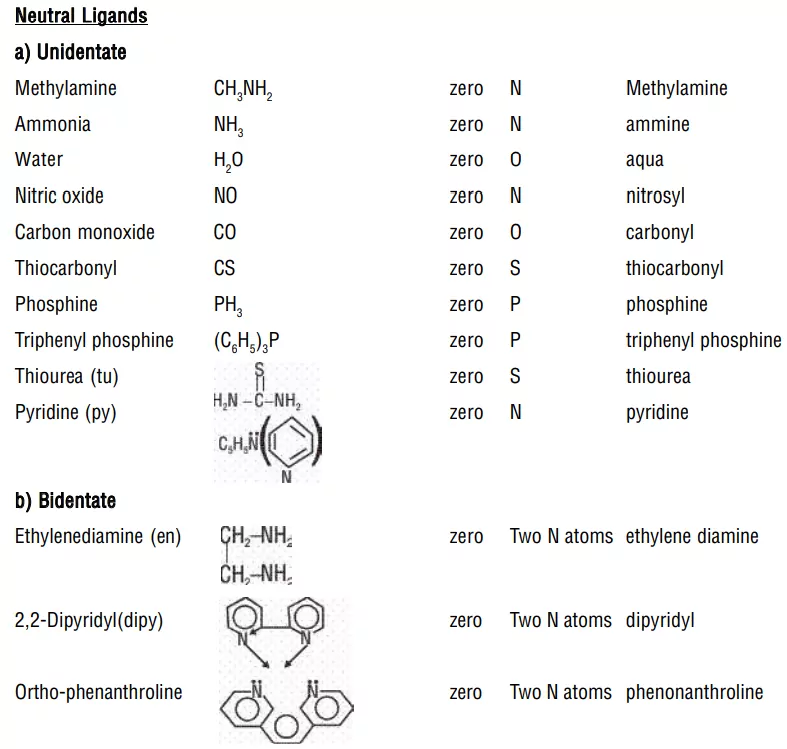
ii) Neutral ligands: These are uncharged and are the electron-pair donor species such as H2O, ROH, NH3, RNH2, R2NH, R3N, etc.
iii) Cationic ligands: They are positively charged and are rare such as NO+, etc.
b) On the basis of denticity: The number of donations accepted by a central atom from a particular ligand is known as the denticity of the ligand.
Based on this, ligands are classified as follows:
i) Monodentate or Unidentate Ligands: A ligand that is bound to a metal ion through a single donor atom. e.g., H2O, NH3, CO, Cl−, NH2−
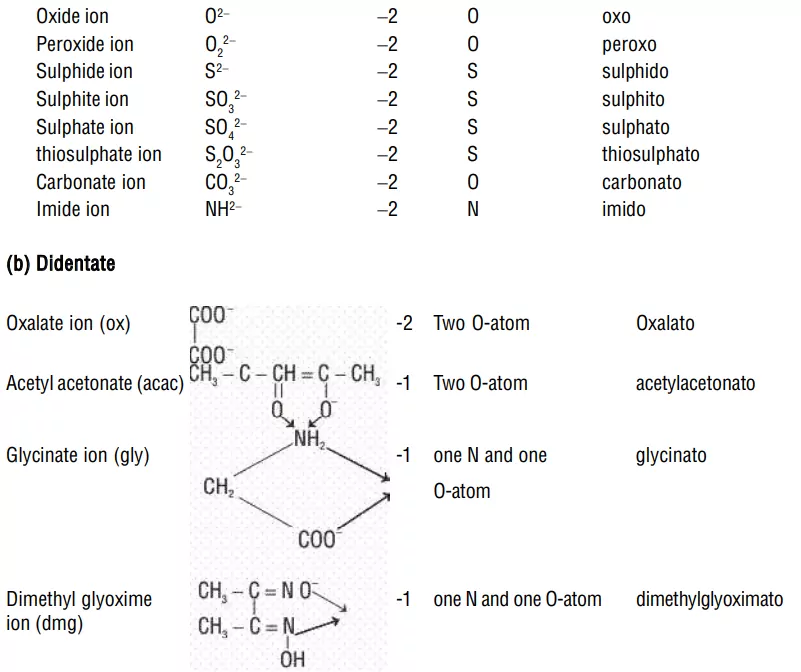
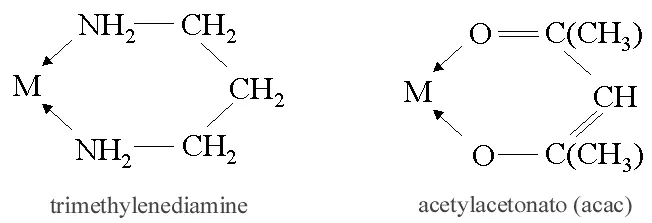
ii) Bidentate (Didentate) ligands: A ligand that binds to the central atom through two donor atoms is called a bidentate ligand. Example: Ethane-1,2-diamine or ethylenediamine (H2NCH2CH2NH2) notated as ‘en’ and oxalate ion (C2O42–) notated as ‘en’.
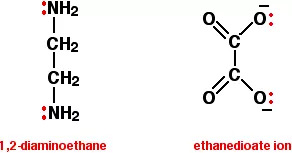
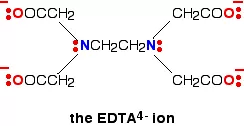
iii) Polydentate ligand: A ligand that binds to the central atom through more than two donor atoms is called a polydentate ligand. For Example Triethylamine ammonia [N(CH2-CH2-NH2)3], Ethylenediamine tetraacetate ion (EDTA4–), etc. Ethylenediamine tetraacetate ion (EDTA4–) is an important hexadentate ligand. It can bind through two nitrogen and four oxygen atoms to a central metal ion.
iv) Ambidentate ligands: They are unidentate ligands that contain more than one donor atom. They can coordinate through two different atoms. Examples of such ligands are the NO2–, CN–, SCN–, CNO–.
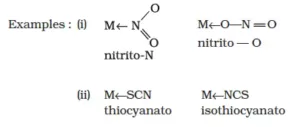 v) Chelating ligands: A ligand that forms a ring structure with the central atom is called a chelating ligand. All polydentate ligands are chelating ligands.
v) Chelating ligands: A ligand that forms a ring structure with the central atom is called a chelating ligand. All polydentate ligands are chelating ligands.
Coordination Compounds Class 12 Notes
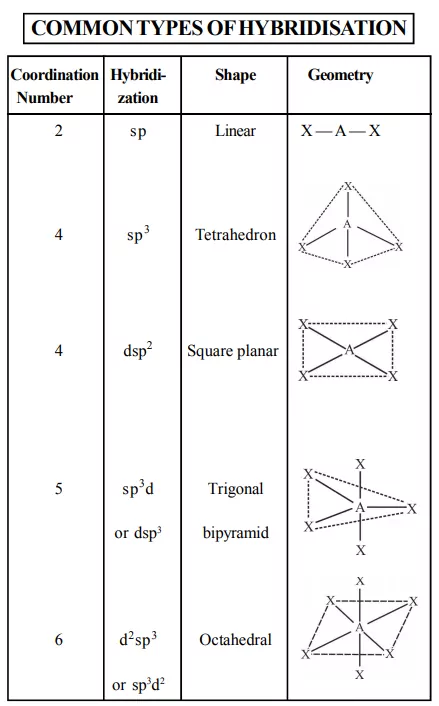
4) Co-ordination number: The number of atoms in a ligand that directly bond to the central metal atom or ion by coordinate bonds is called the coordination number of the metal atom or ion. Some common coordination numbers exhibited by metal ions are 2, 4, and 6. The light transition metals exhibit 4 and 6 coordination numbers while heavy transition metals exhibit coordination numbers above 6.
For example, in the complex ion [PtCl6]2– the coordination number of Pt is 6 and in [Ni(NH3)4]2+, the coordination number of Ni is 4. Similarly, in the complexions, [Fe(C2O4)3]3– and [Co(en)3]3+, the coordination number of both Fe and Co, is 6 because C2O42– and en (ethane-1,2- diamine) are bidentate ligands.
5) Co-ordination sphere: The central metal atom/ion and the ligands directly attached to it are collectively termed as the coordination sphere. The coordination sphere is represented inside square brackets, e.g. [Fe(CN)6]4–.
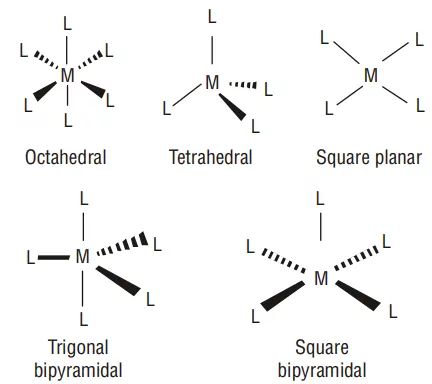
6) Co-ordination polyhedron: The spatial arrangement of the ligands around the central atom/ion defines a coordination polyhedron about the central atom. The most common coordination polyhedra are octahedral, square planar, and tetrahedral. For example, [Co(NH3)6]3+ is octahedral, [Ni(CO)4] is tetrahedral and [PtCl4]2– is square planar.
7) Oxidation Number: The actual charge that a metal atom experiences in a complex are known as its oxidation number. In other words, the oxidation number of a metal atom will be equal to the total charge on this atom if all the ligands are removed without their electron pair. For Example: Find the oxidation number of Co in the complex [Co (en)2 (H2O) (CN)]2+.
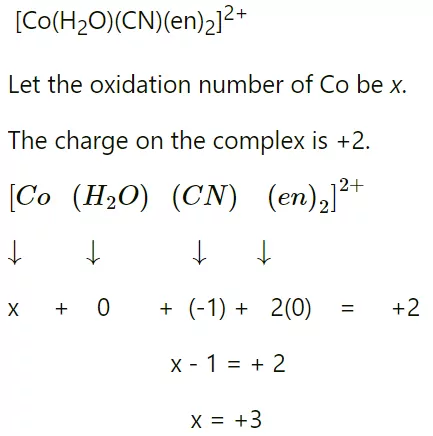
8) Homoleptic and Heteroleptic Complexes: Complexes in which the central metal atom or ion is linked to the only types of ligands are called homoleptic complexes. For example, [Co(NH3)6]3+.
The complexes in which the central metal atom or ion is linked to more than one kind of ligand are called heteroleptic complexes. For example, [Co(NH3)4Cl2]+.
Coordination Compounds Class 12 Notes
Nomenclature of Coordination Compounds Class 12
Werner’s Theory of Coordination Compounds
Alfred Werner, a Swiss chemist proposed a theory about the nature of bonding in the coordination compounds (complexes) in the year 1893. The main postulates of this theory are:
1. Metals possess two types of linkages (valencies).
a) Primary or principal or ionizable links (valency) which are the same as the oxidation state of the metal.
b) Secondary or non-ionizable links (valency) which are the same as the coordination number of the central metal atom/ion. This number is fixed for a metal.
2. A metal atom satisfies both primary as well as secondary linkages (valencies). Primary linkages (valencies) are satisfied by negative ions whereas secondary linkages (valencies) are satisfied by ligands (neutral or negative ions/groups).
3. Ligands satisfying secondary linkages (valencies) are directed towards fixed directions (spatial arrangements) giving a definite geometry to the complex but primary valencies are non-directional. Such spatial arrangements are called coordination polyhedra.
Coordination Compounds Class 12 Notes
Rules for naming mononuclear coordination compounds:
1. Order of naming ions: The positive ion (cation) whether simple or complex, is named first followed by the negative ion (anion). The name is started with a small letter and the complex part is written as one word.
2. Naming ligands:
i) Negative ligands (organic or inorganic) end in −O, eg, CN− (cyano), Cl− (chlorido), Br− (bromido), F− (fluorido), NO2− (nitro), OH− (hydroxo), O2− (oxo), H− (hydrido). If the name of the anionic ligands ends in −ide, −ite or −ate, the last ‘e’ is replaced by ‘O’ giving −ido, −ito and −ato eg, SO42− (sulphato), C2O42− (oxalato), NH2 − (amido), NH2− (imido), ONO− (nitrito).
ii) Neutral ligands have no special ending, NH3 (ammine), H2O (aqua), CO (carbonyl)
iii) Positive ligands (which are very few) end in -ium, NO+ (nitrosoium), NO2+ (nitronium).
3. Numerical prefixes to indicate the number of ligands: If there are several ligands of the same type, the prefixes like di, tri, tetra, penta, and hexa are used to indicate the number of ligands of that type. When the name of polydentate ligand includes a number e.g., ethylenediamine, then bis, tris, tetrakis are used as prefixes.
4. Order of naming of ligands: All ligands whether negative, neutral or positive are named first in alphabetical order followed by the name of the metal atom/ion.
5. Naming of the complexion and ending of the central atom: Ligands are named first followed by the metal atom.
a) If the complexion is a cation or the coordination compound is non-ionic, the name of the central metal ion is written as such followed by its oxidation state indicated by a Roman numeral (such as II, III, IV) in the parentheses at the end of the name of the metal without any space between the two.
b) If the complexion is an anion, the name of the central metal atom is made to end in-ate followed by the oxidation number in brackets without any space between them.
Rules for Writing Formula from the Name of the Mononuclear Complex
(a) Formula of the cation (whether simple or complex) is written first, followed by the anion.
(b) The formula of the complexion (coordination entity) (whether charged or not) is written in square brackets called coordination sphere.
(c) Within the coordination sphere, the symbol of the metal atom is written first followed by the symbols/formulas of the ligands arranged alphabetically according to their names irrespective of the charge present on them. While listing the ligands alphabetically, the following rules should be followed:
i) Polydentate ligands are also listed in alphabetical order.
ii) The position of abbreviated ligands in the alphabetical order is determined from the first letter of the abbreviation.
iii) The position of ligands with special names (such as aqua for water) in alphabetical order is determined from the first letter of the special name.
iv) Abbreviations used for the ligands and the formulas of the polyatomic ligands are enclosed in parentheses separately.
v) The metal atom as well as all the ligands are listed without any space between them.
(d) If the formula of the complexion is to be written without writing the counter ion, the charge on the complexion is indicated outside the square bracket as a right superscript with the number before the sign. For example, [Fe(CN)6]3-, [Cu(NH3)4]2+, etc.
(e) The number of cations or anions to be written in the formula is calculated on the basis that the total positive charge must be equal to the total negative charge, as the complex as a whole is electrically neutral.
Example: Tetraammineaquachloridocobalt(III) chloride has complex ion [Co(NH3)4(H2O)Cl]– and simple ion = Cl—
To balance the charge, the formula will be [Co(NH3)4(H2O)Cl]Cl2.
Coordination Compounds Class 12 Notes
Examples of Nomenclature of Coordination Compounds
1. [Cr(NH3)3(H2O)3]Cl3 – triamminetriaquachromium(III) chloride
Explanation: The complexion is inside the parentheses, which is a cation.
The ammine ligands are named before the aqua ligands according to alphabetical order.
Since there are three chlorides binding with the complexion, the charge on the complexion must be +3 (since the compound is electrically neutral).
From the charge on the complexion and the charge on the ligands, we can calculate the oxidation number of the metal. In this example, all the ligands are neutral molecules. Therefore, the oxidation number of chromium must be the same as the charge of the complexion, +3.
2. [Pt(NH3)5Cl]Br3 – pentaamminechloroplatinum(IV) bromide
Explanation: The complexion is a cation, the counter anion is the 3 bromides.
The charge of the complexion must be +3 since it bonds with 3 bromides.
The NH3 are neutral molecules while the chloride carries -1 charge. Therefore, the oxidation number of platinum must be +4.
3. [Pt(H2NCH2CH2NH2)2Cl2]Cl2 – dichlorobis(ethylenediamine)platinum(IV) chloride
Explanation: ethylenediamine is a bidentate ligand, the bis- prefix is used instead of di-
4. [Co(H2NCH2CH2NH2)3]2(SO4)3 – tris(ethylenediamine)cobalt(III) sulfate
Explanation: The sulfate is the counter anion in this molecule. Since it takes 3 sulfates to bond with two complex cations, the charge on each complex cation must be +3.
Since ethylenediamine is a neutral molecule, the oxidation number of cobalt in the complex ion must be +3.
Again, remember that you never have to indicate the number of cations and anions in the name of an ionic compound.
5. K4[Fe(CN)6] – potassium hexacyanoferrate(II)
Explanation: potassium is the cation and the complexion is the anion.
Since there are 4 K+ binding with a complexion, the charge on the complexion must be -4.
Since each ligand carries a –1 charge, the oxidation number of Fe must be +2.
The common name of this compound is potassium ferrocyanide.
6. Na2[NiCl4] – sodium tetrachloronickelate(II)
Explanation: The complexion is the anion so we have to add the suffix –ate in the name of the metal.
7. Pt(NH3)2Cl4 – diamminetetrachloroplatinum(IV)
Explanations: This is a neutral molecule because the charge on Pt+4 equals the negative charges on the four chloro ligands.
If the compound is [Pt(NH3)2Cl2]Cl2, even then the number of ions and atoms in the molecule are identical to the example, it should be named: diamminedichloroplatinum(II) chloride, a big difference.
8. Fe(CO)5 – pentacarbonyliron(0)
Explanations: Since it is a neutral complex, it is named in the same way as a complex cation. The common name of this compound, iron carbonyl, is used more often.
9. [CrCl2(H2O)4]+ – tetraaquadichlorochromium(III) ion
Explanation – The ligands here are Cl and H2O. Therefore, we will use the monodentate ligand names of “chloro” and “aqua”. Alphabetically, aqua comes before chloro, so this will be their order in the complex’s name. There are 4 aqua’s and 2 chloro’s, so we will add the number prefixes before the names. Since both are monodentate ligands, we will say “tetra[aqua]di[chloro]”.
10. (NH4)2[Ni(C2O4)2(H2O)2] – ammonium diaquabis(oxalato)nickelate(II)
Explanation: The oxalate ion is a bidentate ligand.
11. [Ag(NH3)2][Ag(CN)2] – diamminesilver(I) dicyanoargentate(I).
Some Other Examples:
1. [NiCl4]2−
2. Pt(NH3)2Cl4
3. [Pt(NH3)2Cl2]Cl2
4. [Co(NH3)5CO3]Cl
5. [Cr(H2O)4Cl2]Cl
6. [Zn(OH)4]−2
Answers:
1. Tetrachloridonickelate(II) ion. The complexion, an anion, is inside the parentheses. We have to add the suffix -ate in the name of the metal.
2. Diamminetetrachloroplatinum(IV). This is a neutral molecule. The total charge on the ligands is -4. Therefore, the platinum oxidation number is +4.
3. Diamminedichloroplatinum(II) chloride. Here, the number of ions and atoms are the same. However, the brackets as well as the different oxidation number of the platinum result in a very different name.
4. Pentaamminecarbonatocobalt(III) chloride.
5. Tetraaquadichlorochromium(III) chloride
6. Tetra hydroxide zincate (II) ion.
Werner’s theory could not explain:
a) Only certain elements possess the remarkable property of forming coordination compounds?
b) The bonds in coordination compounds have directional characteristics.
c) Coordination compounds have characteristic magnetic and optical properties.
Coordination Compounds Class 12 Notes
Isomerism in Coordination Compounds Class 12
Isomerism
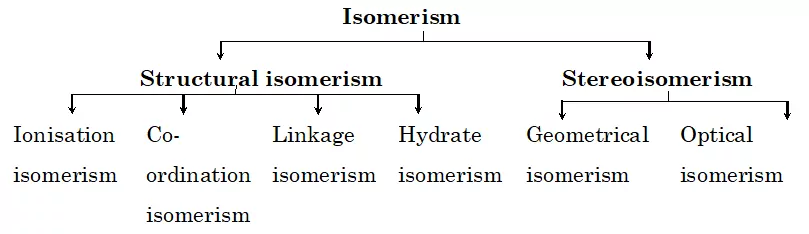
Two or more chemical compounds which have identical chemical formula but different structures are known as isomers and the phenomenon is known as isomerism. The isomers have different arrangements of ligands around the central metal atom. The isomerism shown by co-ordination compounds are broadly divided into two:
Structural Isomerism and Stereo Isomerism.
Structural Isomerism: These are isomers that differ in the structural arrangement of ligands around the central atom. They are of four types:
(a) Ionisation Isomerism
(b) Coordinate Isomerism
(c) Linkage Isomerism
(d) Hydrate Isomerism
(a) Ionisation Isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion. Example: [Co(NH3)5Br]SO4 and [Co(NH3)5 SO4]Br,
[Pt(OH)2(NH3)4]SO4 and [Pt (SO4)(NH3)4](OH)2,
[Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl2.
(b) Coordinate Isomerism: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
Example: [Co(NH3)6] [Cr(C2O4)3] and [Cr(NH3)6] [Co(C2O4)3],
[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
[Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6]
(c) Linkage Isomerism: This isomerism takes place when a monodentate ligand has two possible donor atoms and attaches in two different ways to the central metal atom. Such ligands are known as also ambidentate ligands. For example, nitro (NO2– ) and nitrito (-ONO–), cyano (-CN–), and isocyano (-NC–).
For Example: [Cr(H2O)5SCN]2+ and Cr(H2O)5NCS]2+, [Co(NO2)(NH3)5]Cl2 and [Co(ONO)(NH3)5]Cl2.
(d) Hydrate Isomerism: It is isomerism in which solvent is involved as ligand. If the solvent is water it is called hydrate isomerism. This form of isomerism is also known as ‘solvate isomerism’.
For Example: [Cr(H2O)6]Cl3 (violet) and its solvate isomer [Cr(H2O)5Cl]Cl2.H2O (grey-green).
Coordination Compounds Class 12 Notes
Stereo-isomerism: In stereo-isomerism, the isomers differ only in the spatial arrangement of atoms or groups about the central metal atom. It is also known as space isomerism. It can be further classified into two types:
(i) Geometrical isomerism (ii) Optical isomerism
(i) Geometrical isomerism: This isomerism is due to the difference in the geometrical arrangement of the ligands around the central atom. . It is mainly found in co-ordination complexes with co-ordination numbers 4 (square planar complexes) and 6 (octahedral complexes). When similar ligands are on adjacent positions, it is known as cis form and when these are in the opposite positions, it is known as trans-form. Thus, this is also known as cis-trans isomerism.
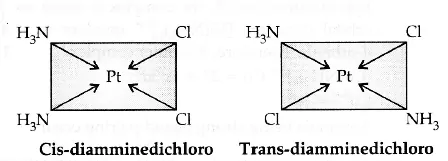
(a) Square planar complex of formula [MX2L2] (X and L are unidentate): The two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer. e.g., [Pt(NH3)2Cl2].
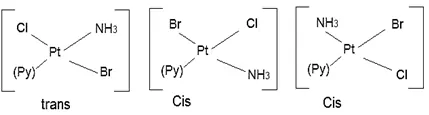
(b) Square planar complex of the type [MABXL] (where A, B, X, L are unidentates): Shows three isomers– two cis and one trans. Such isomerism is not possible for tetrahedral geometry. e.g., [Pt(NH3)(Br)(Cl)(Py)].
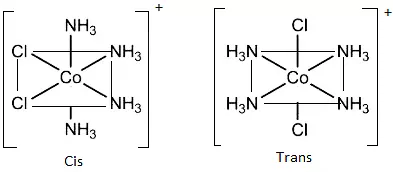
(c) Octahedral complexes of formula [MX2L4]: In which the two ligands X may be oriented cis or trans to each other. e.g., [Co(NH3)4Cl2]+
(d) Octahedral complexes of formula [MX2A2]: Where X is unidentates and A are bidentate and form cis and trans isomers. e.g., [CoCl2(en)2].

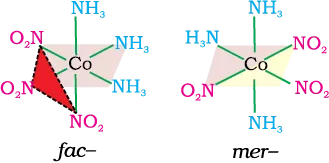
(e) Octahedral coordination entities of the type [MA3B3] Like [Co(NH3)3(NO2)3]: If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac) isomer. When the positions are around the meridian of the octahedron, we get the meridional (mer) isomer.
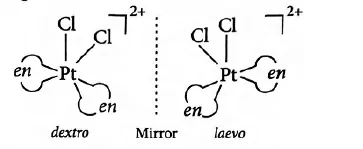
(ii) Optical Isomerism: Optical isomers are mirror images that cannot be superimposed on one another. These are also called enantiomers. The molecules or ions that cannot be superimposed are called chiral. There are two forms of optical isomers – Dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right, l to the left). Optical isomerism is common in octahedral complexes involving bidentate ligands. In a co-ordination entity of the type [PtCl2(en)2]+, only the cis-isomer shows optical activity. The trans-isomer has a plane of symmetry and is optically inactive.
[M(AA)3] type, [M(AA)2B2] or [M(AA)2BC] type and [M(AA)B2C2] type
Square planar complexes: Generally square planar complexes are not optically active as they have all the ligands and metal atoms in one plane. That is why there is a plane of symmetry.
Coordination Compounds Class 12 Notes
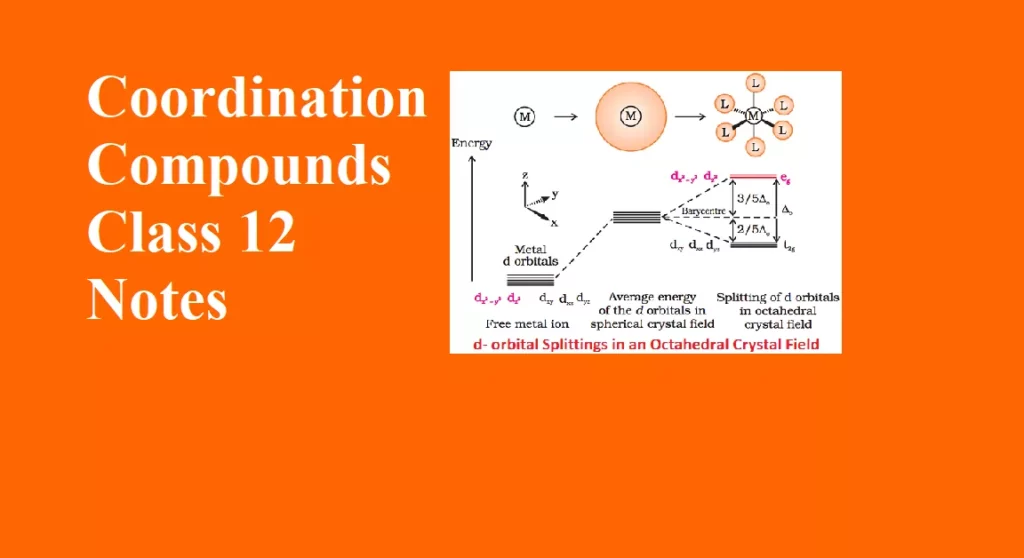
Valence Bond Theory Coordination Compounds
Valence Bond Theory
The bonding in coordination compounds can be explained by Valence Bond Theory (VBT). It was developed by Pauling. This theory mainly deals with the electronic structure of central metal ion, geometry, magnetic properties of complex, kind of bonding. According to this theory, the metal atom or ion under the influence of ligands form inner orbital and outer orbital complex. These hybridized orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
The main features of the valence bond theory are summarized below:
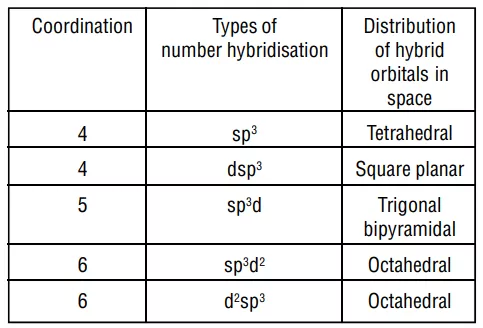
1. Inner orbital complex: When the complex formed involved inner (n-1)d orbitals for hybridisation (d2sp3 ). Electrons are made to pair up so complex will be either diamagnetic or has fewer unpaired electrons. It is a low spin complex. Eg. [V(H2O)6]3+, [Co(NH3)6]3+.
2. Outer orbital complex: When the complex formed uses outer d orbitals for hybridisation, (sp3d2), it has a large number of unpaired electrons. It is a high spin complex. Eg. [CoF6]3-, [Fe(H2O)6]3+.
FORMATION OF OCTAHEDRAL COMPLEXES
Example: Inner orbital complex, [Co(NH3)6]3+
Co→ [Ar]3d74s2
Co+3→ [Ar]3d64s0
With a +3 oxidation state NH3 act as a strong ligand.
All electrons are paired therefore, complex will be diamagnetic in nature.
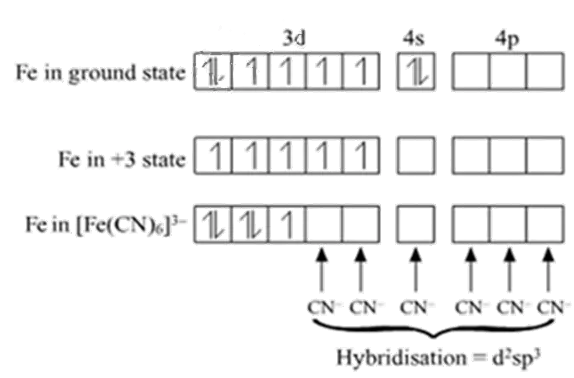
Example: Inner orbital complex, [Fe(CN)6]3-
Fe → [Ar]3d64s2
Fe+3→ [Ar]3d54s0
With +3 oxidation state CN act as a strong ligand.
FORMATION OF SQUARE PLANAR COMPLEXES
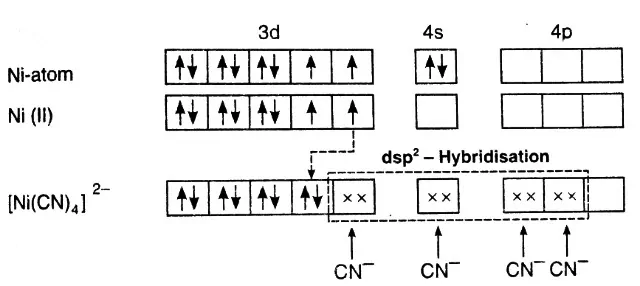
Example: Inner orbital complex, [Ni(CN)4]2-:
Ni – 3d8, 4s2
Ni2+ – 3d8, 4s0
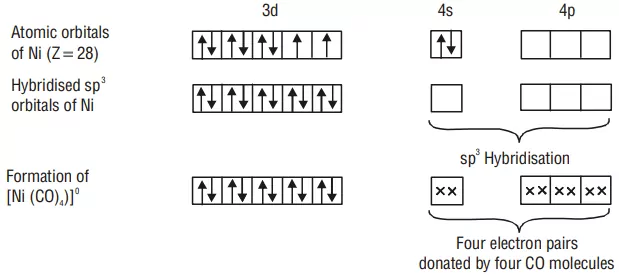
FORMATION OF TETRAHEDRAL COMPLEXES
Example: Inner orbital complex [Ni(CO)4]:
Outer orbital complexes
i). In these complexes s, p, and d orbitals which are involved in hybridization, belong to the highest quantum number (n).
ii) Complex compound formed by the use of outer n and d orbitals will be paramagnetic.
iii) Outer orbital complexes are also known as high-spin or spin-free complexes.
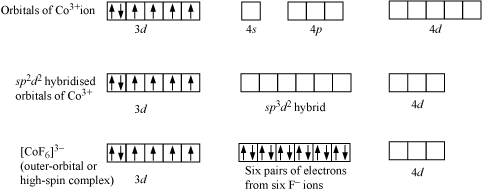
iv). The outer orbital complexes have a high number of unpaired electrons, E.g. [CoF6]3–
For Example: Outer orbital complex [CoF6]3–
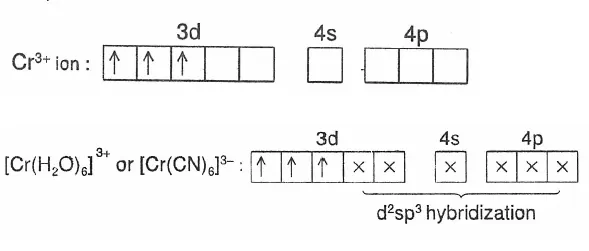
For Example: Outer orbital complex [Cr(H2O)6]3+:
Coordination Compounds Class 12 Notes
Limitations of Valence Bond Theory:
Even though the valence bond theory explains the formation, structures, and magnetic behavior of coordination compounds to a larger extent, it suffers from the following shortcomings:
i). It does not explain the relative energies of different structures.
ii). It offers no explanation of the colour observed for the complexion.
iii). It cannot predict whether a 4-coordinate complex will be tetrahedral or square planar.
iv). It does not take into account the splitting of d-energy levels. v. It does not explain the spectra of complexes.
Coordination Compounds Class 12 Notes