CARBON AND ITS COMPOUNDS
Carbon plays a very important role for all living beings. The amount of carbon in the earth’s crust is merely 0.02%, which is available in the form of minerals such as carbonates, hydrogen-carbonates, coal, and petroleum. The presence of carbon in the atmosphere of the earth is 0.03%, in the form of carbon dioxide. Almost all carbon compounds (except a few) are poor conductors of electricity. Two essential properties of Carbon are Catenation and tetravalency. Diamond and graphite both are formed by carbon atoms; however, the difference lies between them in the manner in which the carbon atoms are bonded to one another. In a diamond, each atom of carbon is bonded to four other carbon atoms and forms a rigid three-dimensional structure. In graphite, each atom of the carbon is bonded to three other carbon atoms in the same plane, which gives a hexagonal array.
There is also a difference in some physical structures of diamond and graphite. Diamond is the hardest substance known whereas graphite is a smooth and slippery substance. Graphite is a good conductor of electricity whereas diamond is not.
The saturated hydrocarbons are known as alkanes. The unsaturated hydrocarbons, which comprise one or more double bonds, are known as alkenes. The unsaturated hydrocarbons, which comprise one or more triple bonds, are known as alkynes.
Important Terms:
Organic Compound: Organic compounds are the one which is made up of carbon and hydrogen.
CARBON
Introduction:
Compounds of carbon are known as organic compounds. All organic compounds contain hydrogen along with carbon. Since they are fundamental organic compounds, they are also known as hydrocarbons. The study of carbon compounds such as hydrocarbons and their derivatives is called organic chemistry.
Bonding in Carbon:
The carbon atom has four electrons in its outermost shell. It requires four electrons to achieve the stable, 8 electrons, inert gas configuration. Carbon atoms can only achieve the inert gas electron arrangement by sharing their electrons. Hence, carbon always forms covalent bonds. The valency of carbon is four since one carbon requires 4 electrons to achieve the nearest inert gas configuration. Thus, we can say that carbon is tetravalent. The four valencies of carbon are usually represented by drawing four short lines around the symbol of carbon (C).
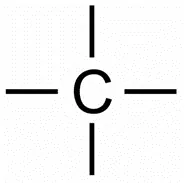
Allotropes of Carbon:
The various physical forms in which an element can exist are called the allotropes of that element.
Carbon has three allotropes:
→ Crystalline forms such as diamond and graphite
→ Amorphous forms such as coal, charcoal, lamp black, etc.
→ Fullerenes
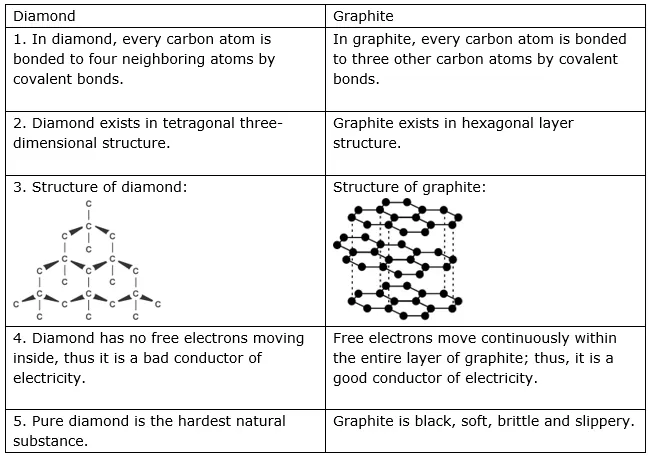
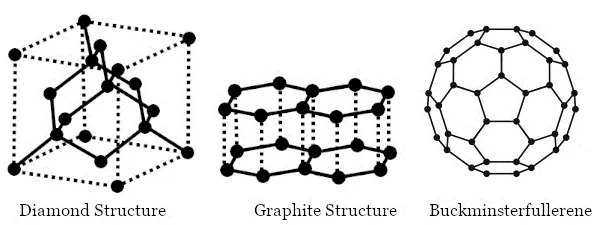
Diamond: In a diamond, each carbon atom is bonded to four other carbon atoms, forming a three-dimensional structure. The rigid structure of a diamond makes it a very hard substance. It is a non-conductor of electricity since there are no free electrons in a diamond crystal. It can be synthesized by subjecting pure carbon to very high pressure and temperature.
Graphite: In graphite, each carbon atom is bonded to three other carbon atoms in the same plane, giving a hexagonal array. One of the bonds is a double bond and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers, one above another. Graphite is smooth and slippery. It is a very good conductor of electricity due to the presence of free electrons.
Fullerene: It is an allotrope of carbon-containing clusters of 60 carbon atoms joined together to form spherical molecules. There are 60 carbon atoms in a molecule of buckminsterfullerene, so its formula is C60. The allotrope was named buckminsterfullerene after the American architect Buckminster Fuller.
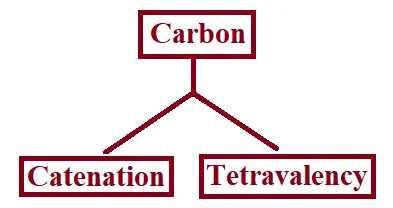
Versatile Nature of Carbon:
The two characteristic properties of the element carbon which lead to the formation of a very large number of organic compounds are:
→ Catenation: The property of the element carbon due to which its atoms can join one another to form long carbon chains is called catenation.
→ Tetravalency: Carbon has a valency of four. So, it is capable of bonding with four other atoms of carbon or atoms of some other monovalent element.
Electron dot structure:
Formation of hydrogen molecule (Single Bond) – The atomic number of hydrogen is 1. Hence it has one electron in its K shell and it requires one more electron to fill the K shell. So, two hydrogen atoms share their electrons to form a molecule of hydrogen, H2.
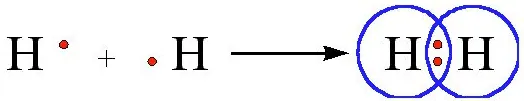
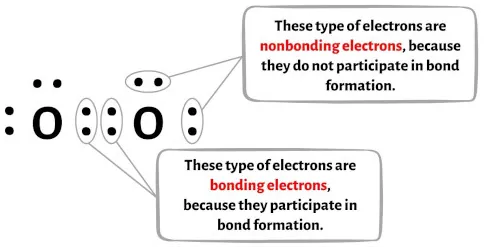
Formation of oxygen molecule (Double Bond): The atomic number of oxygen is 8. So, it has six electrons in its L shell and it requires two more electrons to complete its octet. So, each atom of oxygen shares two electrons with another atom of oxygen. The two electrons contributed by each oxygen atom give rise to a double bond between the two atoms.
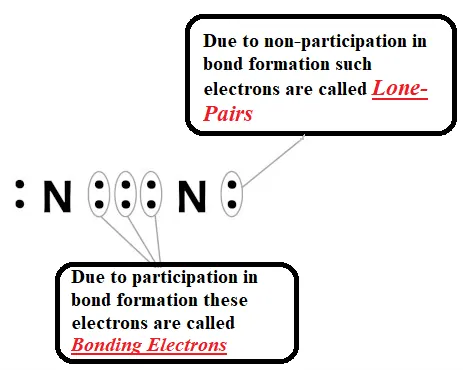
Formation of nitrogen molecule (Triple Bond): Nitrogen has the atomic number 7. So, it has five electrons in its L shell and it requires three more electrons to complete its octet. So, in order to attain an octet, each nitrogen atom in a molecule of nitrogen contributes three electrons giving rise to three shared pairs of electrons that result in a triple bond between the two atoms.
Classification of Hydrocarbons:
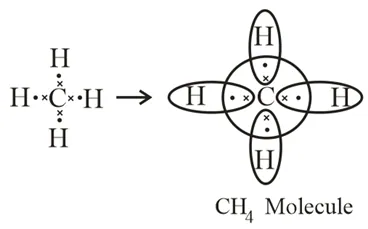
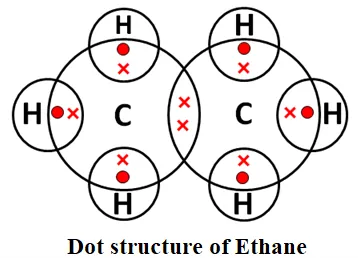
→ Saturated Hydrocarbons: Hydrocarbons in which the carbon atoms are connected by only single bonds are called saturated hydrocarbons. Saturated hydrocarbons are called alkanes. The general formula of alkanes: CnH2n+2, n = the number of carbon atoms. Methane and ethane are saturated hydrocarbons, which contain only carbon-carbon single bonds. The electron dot structure of methane and ethane is given below:
Unsaturated Hydrocarbons (Alkenes and Alkynes): Hydrocarbons in which two carbon atoms are connected by a double or a triple bond are called unsaturated hydrocarbons. Unsaturated hydrocarbons are of two types:
→ Alkenes
→ Alkynes
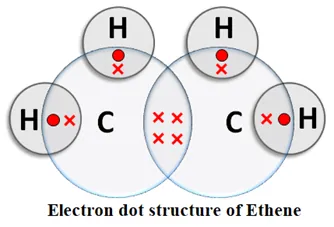
Alkenes: An unsaturated hydrocarbon in which two carbon atoms are connected by a double bond is called an alkene. Alkenes contain the > C = C < group. General formula: CnH2n, where n = number of carbon atoms
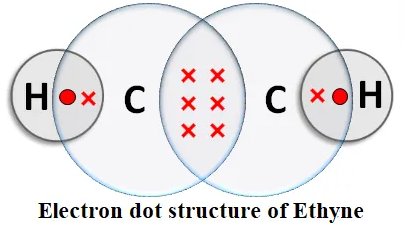
Alkynes: An unsaturated hydrocarbon in which two carbon atoms are connected by a triple bond is called an alkyne. An alkyne contains the -C≡C- group. General formula: CnH2n-2, where n = the number of carbon atoms.
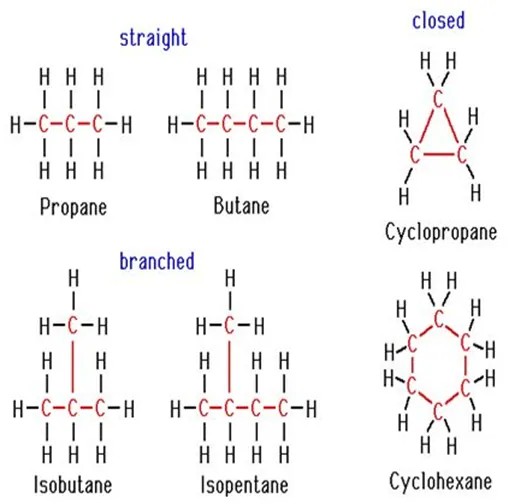
Chains, Branches, and Rings: Carbon Compounds can be classified as straight-chain compounds, branched chain compounds, and cyclic compounds. They are represented as –
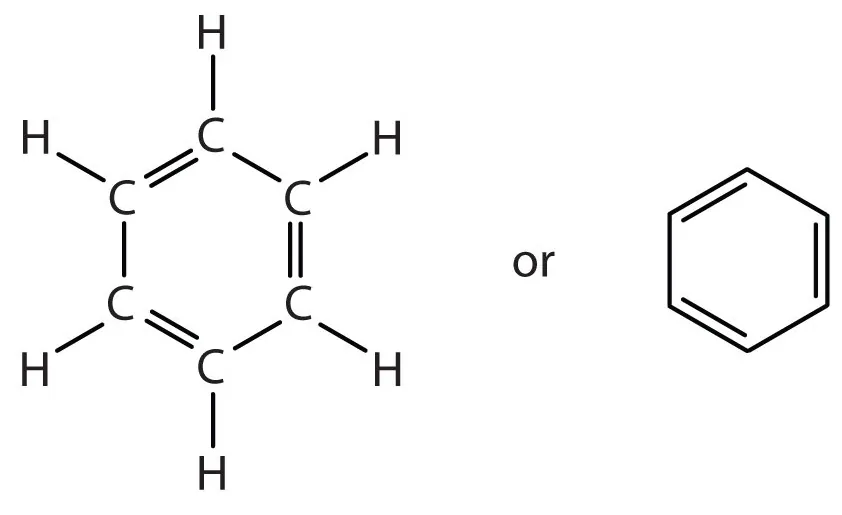
Structure of Benzene:
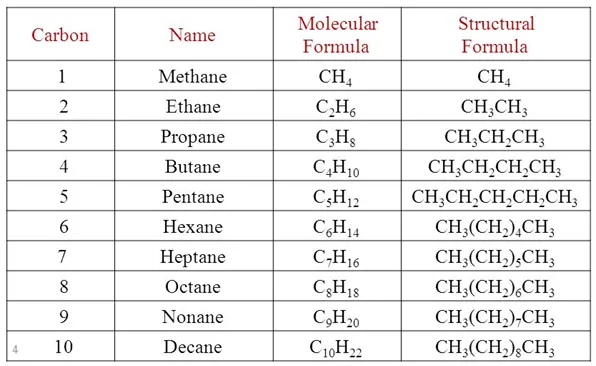
Name, Formulae, and Structures of Saturated Compounds of Carbon and Hydrogen
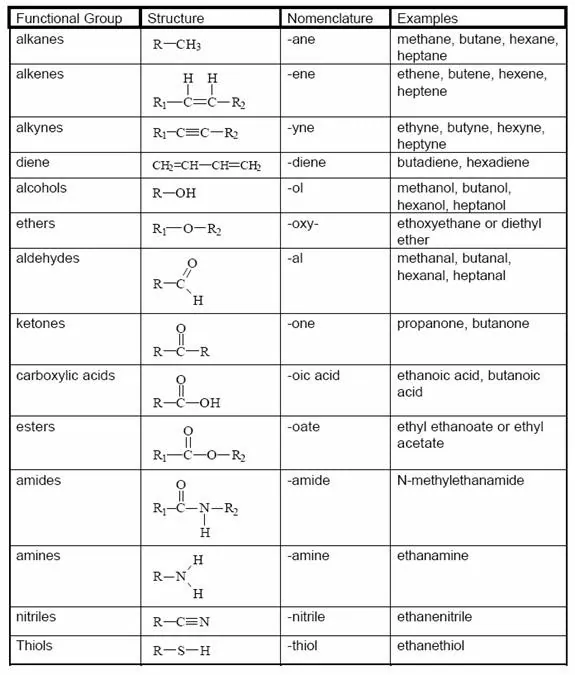
Functional group: An atom or group of atoms which provides certain characteristic properties to the compound, is known as a functional group. The functional group is attached to the carbon chain through the valency by replacing one hydrogen atom or atom.
Homologous Series:
It is a group of organic compounds having a similar structure and chemical properties in which the successive compounds differ by a -CH2 group. Each member of the series differs from the preceding one by the addition of a -CH2 group and by 14 a.m.u. All members of a homologous series have the same general formula. The physical properties of the members show a gradation in properties as their molecular mass increases. The chemical properties also show a gradient similarity.
Carbon and Its Compounds


