Electronegativity Ionisation and Electron Gain Enthalpies
Electronegativity Ionisation and Electron Gain Enthalpies:
Ionization Enthalpy (∆iH): Minimum amount of energy required to remove the most loosely bound valence electron from an isolated gaseous atom so as to convert it into gaseous cation.
It may be represented as: X(g) + ∆iH → X+(g) + e–
Its unit is kJ/mol or J/mol.

The energy required to remove the first electron from the outermost shell of a neutral atom is called first ionization enthalpy (∆iH1)
X(g) + ∆iH1 → X+(g) + e–
Second Ionisation enthalpy (∆iH2) is the amount of energy required to remove an electron from a unipositive ion.
X+(g) + ∆iH2 → X2+(g) + e–
Energy is always required to remove an electron from an atom or ion. So ∆iH is always positive. The second ionization enthalpy is always higher than the first ionization enthalpy. This is because it is more difficult to remove an electron from a positively charged ion than from a neutral atom.
Similarly, the third ionization enthalpy is higher than the second ionization enthalpy, and so on. i.e. ∆iH1 < ∆iH2 < ∆iH3………… As the ease of removal of electrons increases, the ionization enthalpy decreases.
Factor affecting Ionization enthalpy: The important factors which affect ionization enthalpy are:
a) Atomic size: Greater the atomic size (atomic radius), the smaller will be the ionization enthalpy.
b) Nuclear charge: The value of ionization enthalpy increases with nuclear charge.
c) Shielding / Screening effect of electrons: As the shielding effect of inner electrons increases, ionization enthalpy decreases. In multi-electron atoms, electrons in the valence shell experience an attractive force from the nucleus and repulsive force from electrons in the inner shells. As a result, the attractive force exerted by the nucleus on the valence shell electrons gets reduced by the repulsive force exerted by the inner shell electrons.

d) Presence of half-filled or completely filled orbitals increases ionization enthalpy.
e). Penetration effect of electrons: IE increases as the penetration effect of electrons increases. s > p > d > f. If the penetration effect of the electron is more, it will be closer to the nucleus, hence it is more firmly held by the nucleus.
Electron Gain Enthalpy (∆egH): It is the enthalpy change when an electron is added to an isolated gaseous atom. X(g) + e– → X– (g). Its unit is kJ/mol.
The greater the amount of energy released, the higher is the electron gain enthalpy of the element.
It may be positive or negative depending on the nature of the element. For most of the elements, energy is released when an electron is added to its atoms. So ∆egH is negative. Noble gases have large positive electron gain enthalpy because of their completely filled (stable) electronic configuration.

Electronegativity Ionisation and Electron Gain Enthalpies
Variation along the Period: From left to right across a period, ∆egH becomes more negative. This is because of a decrease in atomic radius and an increase in nuclear charge. So, the ease of addition of electrons increases and hence the ∆egH.
Variation down the Group: As the size increases, the tendency to add the electron decreases hence electron gain enthalpy becomes less – ve.
Electron gain enthalpy of fluorine is less negative than chlorine. This is because, when an electron is added to F, it enters into the smaller 2nd shell. Due to the smaller size, the electron suffers more repulsion from the other electrons. But for Cl, the incoming electron goes to the larger 3rd shell. So, the electronic repulsion is low and hence Cl adds electron more easily than F.
Thus, in the modern periodic table, alkali metals have the least –ve ∆egH, and halogens have the most –ve ∆egH. Among halogens, the negative ∆egH decreases as follows. Cl> F > Br > I. The negative electron gain enthalpy is also called electron affinity.
Noble gases have positive electron gain enthalpy. They have completely filled orbitals. Additional electrons will be placed in the next higher shell. As a result, energy has to be supplied to add an additional electron.
The formation of O is exothermic but O is endothermic.
O (g) + e– → O– (g); ∆egH = – 141 KJ mol
O– (g) + e– → O2- (g); ∆egH = + 780 KJ mol
∆egH After the addition of one electron the atom becomes negatively charged and the second electron is to be added to a negatively charged ion. The addition of a second electron is opposed by electrostatic repulsion, hence energy has to be supplied for the addition of a second electron to overcome the strong electrostatic repulsion between the negatively charged O ion and the second electron is added.
Electronegativity Ionisation and Electron Gain Enthalpies
Electronegativity: The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is termed as its electronegativity. It is not a measurable quantity and so it has no unit. There are different scales for measuring the Electronegativity of elements. The most commonly used is the Pauling Electronegativity scale developed by Linus Pauling.
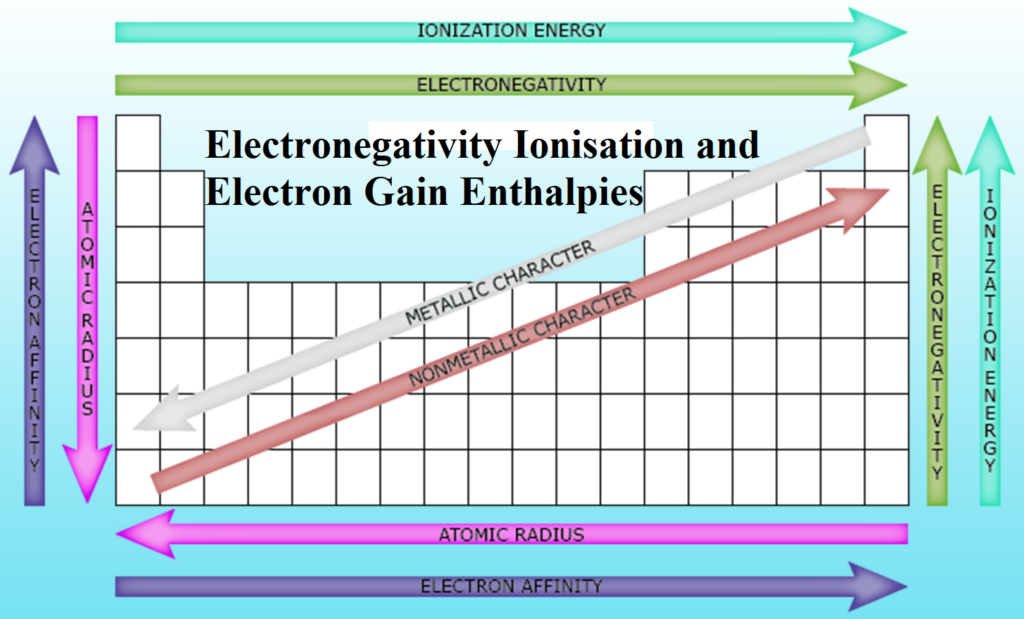
(i) In period- The electro-negativity increases from left to right in a period.
(ii) In group- The electro-negativity decreases from top to bottom in a group.

Difference between Electronegativity and Electron Gain Enthalpy:

Diagonal Relationship: Some elements of the second period show similarities with elements of the third period placed diagonally to each other due to the same charge/radius ratio.

Anomalous Behaviour of the first element of every group is due to
i). Small size and high electronegativity: N can form p π – p π multiple bonds whereas P cannot.
ii) High IE: They form only covalent compounds and not ionic compounds.
iii) Absence of vacant d orbitals: N cannot form NCl5 or R3N = O since it cannot expand its covalence beyond 4 whereas P can form PCl5 and R3P = O.
Valence Electrons: The electrons present in the outermost shell are called as valence electrons. Because the electrons in the outermost shell determine the valency of an element.
Valency of an Element: According to the electronic concept of valency, “the number of electrons which an atom loses or gains or shares with another atom to attain the noble gas configuration is termed as its valency.”
Periodicity:
(i) In period- The valency first increases then decreases from left to right in a period.
(ii) In group- The valency remains constant from top to bottom in a group.

Electropositivity: It is the tendency of an atom to lose the most loosely bound electron (valence electron). It is directly related to the metallic character of elements. It depends on the atomic size and nuclear charge. As the atomic radius increases, electropositivity increases.
Along a period, electropositivity decreases from left to right. But down a group, it increases. So, francium is the most electropositive element and fluorine is the least electropositive element.
Electronegativity Ionisation and Electron Gain Enthalpies
Variation of Various Properties along with the Periods and Groups


Nomenclature of Elements with Atomic Number > 100: It was decided by IUPAC that the names of elements beyond atomic number 100 should use Latin words for their numbers. The names of these elements are derived from their numerical roots.
Numerical -> 0 1 2 3 4 5 6 7 8 9
Roots nil un bi tri quad pent hex sept oct en

Electronegativity Ionisation and Electron Gain Enthalpies
Classification and Blocks in Periodic Table