Que. Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l). What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl? Ans. 0.75 M means 0.75 mol of HCl is present in 1 L of solution 1 Mol of HCl weighs = 1 + 35.5 = 36.5 g 0.75 mol of HCl weighs = 0.75 × 36.5 = 27.37 g ⸫ 27.37 g of HCl is present in 1000 ml (1 L) of solution 1000 ml of solution…
Author: Dr. Vikas Jasrotia
CBSE Sample Paper Class 10 Science (2022-23) Central Board of Secondary Education (CBSE) has released the Sample Papers 2023 for the Class 10 board examination. Sample papers are one of the best resources for learners during revision and preparation for the final board exam. The sample papers are now available on the official websites cbseacademic.nic.in and cbse.gov.in. By doing the practice of these sample papers, students can get a good idea and knowledge of the paper pattern and the way to write the answers. CBSE Sample Papers lay out the paper pattern, the kind of questions and the choices, etc.,…
CBSE Sample Paper Session 2022-23 Chemistry Central Board of Secondary Education, CBSE has publicized the CBSE Sample Papers 2023 for the Class 12 board examination. Sample papers or model papers are one of the best resources for learners during revision and preparation for the final board exam. The sample papers are now available on the official websites cbseacademic.nic.in and cbse.gov.in. By practicing these, students can get a good idea of the paper pattern. CBSE Sample Papers lay out the paper pattern, the kind of questions and the choices, etc., that would form the question papers of the CBSE Board Examinations…
Matter in Our Surroundings Class 9 MCQ – Solved Matter in Our Surroundings Class 9 MCQ – Solved Que 1. The quantity of matter present in an object is called its: (a) Weight (b) Gram (c) Mass (d) Density Ans 1. (c) Mass Reason: Mass depends on the quantity of matter. Que 2. Which of the following phenomena would increase on raising the temperature? (a) Diffusion, evaporation, compression of gases (b) Evaporation, compression of gases, solubility (c) Evaporation, diffusion, expansion of gases (d) Evaporation, solubility, diffusion, compression of gases Ans 2. (c) Evaporation, diffusion, expansion of gases Que 3. In…
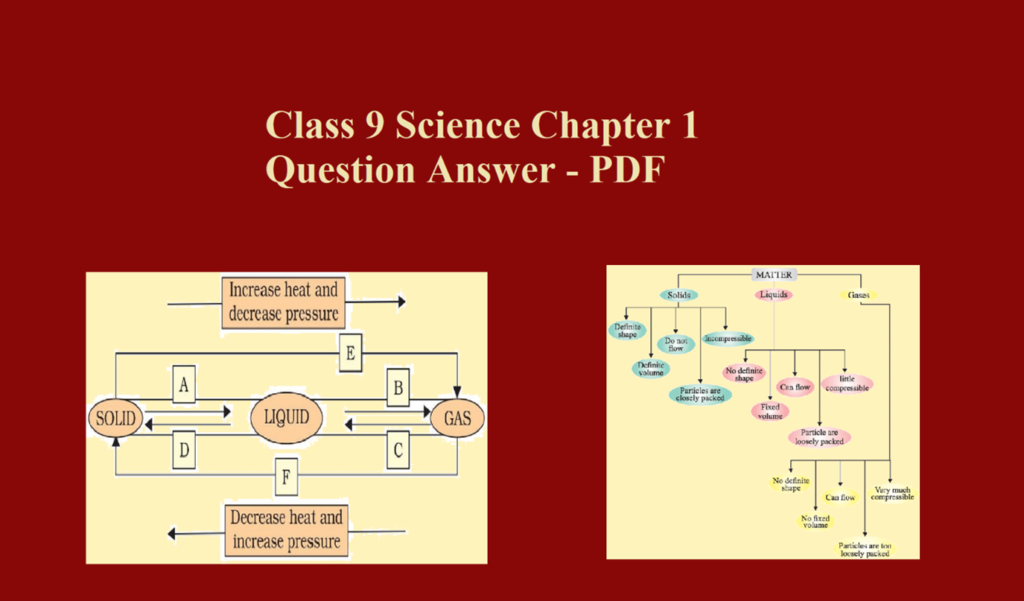
Class 9 Science Chapter 1 Question Answer – PDF The post Class 9 Science Chapter 1 Question Answer – PDF contains important questions from chapter Matter in Our Surroundings of class 9 science. PDF is available at the end of this post. Que 1. Which of the following are matter? Chair, air, love, smell, hate, almonds, thought, cold, cold drink, the smell of perfume. Ans 1. Chair, air, almond, cold-drinks Que 2. What happens to the sugar when it dissolves in water? Where does the sugar go? What information do you get about the nature of matter from the dissolution…
Haloalkanes and Haloalkanes Class 12 – Questions & Answers Haloalkanes and Haloarenes Class 12 Que 1. Give reasons for the following: (i) The bond length of the C–Cl bond is larger in haloalkanes than that in haloarenes. (ii) Although alkyl halides are polar in nature but are not soluble in water. (iii) Haloalkanes react with KCN to form alkyl cyanide as the main product while with AgCN alkyl isocyanide is the main product. (iv) Sulphuric acid is not used in the reaction of alcohol with Kl. (v) Thionyl chloride is the preferred reagent for converting ethanol to chloroethane.…
Some Basic Concepts of Chemistry NEET Questions PDF Some Basic Concepts of Chemistry NEET Questions PDF Que 1. Why is the law of Gay-Lussac not obeyed if any reactant or product is not a gas? Ans 1. If any reactant or product is a liquid, the volume occupied by a liquid is extremely small and hence, the law is not obeyed. Que 2. If the speed of Shatabdi express is 150 miles per hour, then express its speed in SI unit. Ans 2. Que 3. A battery acid contains 24.5% by mass of H2SO4. What is the molality of the…
Some Basic Concepts of Chemistry Class 11 – MCQs (Answers) Ans 1. Ans 2. 6.023 × 1023 has four significant figures. The total number of digits in a number including the last digit whose value is uncertain is called the number of significant figures. Ans 3. All non-zero digits are significant. Hence, there are 6 significant figures in 10.3406 g. Ans 4. 0.02856 × 298.15 × 0.112 /0.5785 Ans 5. Precision refers to the closeness of various measurements for the same quantity. Accuracy is the agreement of a particular value to the true value of the result. Thus, A and…
Matter in Our Surroundings Notes Class 9 The post Matter in Our Surroundings Notes Class 9 involve following topics: Introduction Composition of Matter Matter and Properties of Matter Diffusion States of Matter Rigidity & Fluidity Interconversion of the state of Matter Effect of change of temperature & pressure Latent Heat Latent heat of vaporization and Fusion Sublimation, Evaporation, Cooling Effect INTRODUCTION: ◊ Everything in this universe is made of materials that scientists named‘ matter’. ◊ A pencil, a pen, a book, the food we eat, the clothes we wear, the walls of our houses, doors– everything around is made up…
Some Basic Concepts of Chemistry Class 11 -MCQs Some Basic Concepts of Chemistry Class 11 1. If the measured temperature on the Fahrenheit scale is 200°F then the reading on the Celsius scale will be ……. (a) 40°C (b) 94°C (c) 93.3°C (d) 30°C 2. Which one of the following data has only four significant figures? (a) 6 023 × 1023 (b) 285 cm (c) 0.0025 L (d) 0.200 g 3. The number of significant figures in 10.3406 g is …… (a) 2 (b) 3 (c) 1 (d) 6 4. The result of which of the following has/have the least…