Answers Assignment – 1 Chemical Reactions and Equations All the subjective answers of the previous post are provided in the post Answers Assignment 1 – Chemical Reactions and Equations Ans 1. (i) Decomposition reaction (ii) Double displacement reaction Ans 2. Silver does not evolve hydrogen on reacting with dil. H2SO4 as silver is less reactive metal than hydrogen. Ans 3. Diamond and graphite are the two allotropes of carbon but they do not evolve the same amount of heat on combustion because they differ in the arrangement of carbon atoms and also their shapes one different. Ans 4. The oxidizing agent supplies the oxygen…
Author: Dr. Vikas Jasrotia
Assignment – 1 Chemical Reactions and Equations This subjective Assignment is basically focused to check the reasoning and logical aptitude of the students. Assignment – 1 Chemical Reactions and Equations also focused to cover wide portion of chapter. Identify the type of chemical reaction …
The Effect of Oxidation-Reduction in Daily Life Corrosion Many metals are chemically active elements and get easily affected by substances like moisture, air, acids, etc and undergo Oxidation, Reduction in Daily Life. Corrosion – The process of slow conversion of metals into their undesirable compounds due to their reaction with oxygen, water, acids, gases etc. present in the atmosphere is called corrosion. Rusting of Iron: We know iron articles that are shiny when new and gets coated with a reddish-brown powder when left for some time this process is called Rusting of Iron. Chemically, Rust is a hydrated ferric oxide (Fe2O3.xH2O) Advantages of Corrosion:…
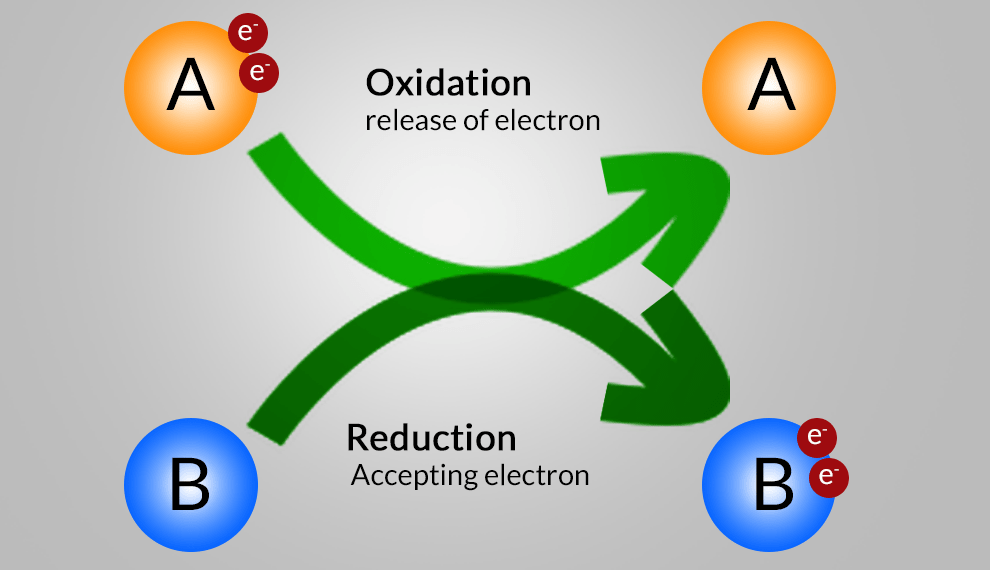
Redox Reaction, Oxidation and Reduction Reactions with exact equations This part of the types of chemical reaction of chapter Chemical Reactions and Equations involve the types Redox Reaction, Oxidation and Reduction Reactions with exact equations. v). Redox Reaction: A reaction in which reduction and oxidation takes place simultaneously. ZnO (s) + C (s) → Zn (s) + CO (g) Zinc Carbon Zinc Carbon Oxide Monoxide vi). Oxidation Reaction: Oxidation is the gain of oxygen. For example: 2…
Combination, Decomposition, Displacement And Double Displacement reactions Types of Chemical Reactions: i). Combination Reaction ii). Decomposition Reaction a). Thermal Decomposition b). Electrolytic Decomposition iii). Displacement Reaction OR Substitution reaction iv). Double Displacement Reaction a). Precipitation Reaction b). Neutralisation Reaction v). Redox Reaction vi). Oxidation Reaction vii). Reduction Reaction viii). Endothermic Reaction ix). Exothermic Reaction Explanation of the types of Chemical reactions i.e Combination, Decomposition, Displacement And Double Displacement reactions using examples: i). Combination Reaction: The reactions in which two or more substances combine to…
Chemistry Balancing Chemical Equations Practice Balanced Equation A balanced equation is one in which the number of atoms on the reactant and product sides is equal. Or A number of atoms of an element on the reactant side = number of atoms of that element on the product side. The simple form of representation of a chemical reaction in words is known as a word equation. Magnesium + Oxygen ———–> Magnesium Oxide Representation of a chemical reaction with the help of chemical formula is called a chemical equation. 2Mg + O2→ 2MgO Chemistry Balancing Chemical…
Chemical Reactions And Equations Notes Physical Change Change in physical properties. Melting Boiling Condensation Note- No change occurs in the identity of the substance Chemical Change Atoms in the reactants are rearranged to form one or more different substances. Old bonds are broken, new bonds are formed. Reactants lose their properties to form products of different properties. Examples – Cooking of food Rusting of iron Heating of Lead nitrate Souring of milk Ripening of fruit. Rusting of iron is a chemical change because A new substance iron oxide is formed. The change is permanent; the article has got a rust layer (which may only peal off). There is an increase in mass when rust forms. An energy change…