Acids Bases and Salts Important Questions Acids Bases and Salts Important Questions contain the remaining questions of the chapter: Que 21. Compounds such as alcohol and glucose also contain hydrogen but are not categorized as acids. Describe an activity to prove it. Que 22. Why does distilled water not conduct electricity, whereas rainwater does? Que 23. Why do acids not show acidic behavior in the absence of water? Que 24. Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test…
Author: Dr. Vikas Jasrotia
Name Reactions & Physical Properties Haloalkanes Name reactions & Physical Properties Haloalkanes 1). Halogen Exchange: (a). Finkelstein reaction: Alkyl chlorides or bromides when treated with NaI in dry acetone, alkyl iodides are formed. This reaction is known as the Finkelstein reaction. R-X + NaI → R-I + NaX (where X = Cl, Br) CH3CH2Cl + NaI → CH3CH2I + NaCl (b). Swarts reaction: This method is used for the preparation of alkyl fluorides. Here alkyl chloride or bromide is treated with a metallic fluoride like AgF, Hg2F2, CoF2 or SbF3, to get alkyl fluoride. R-X + AgF → R-F +…
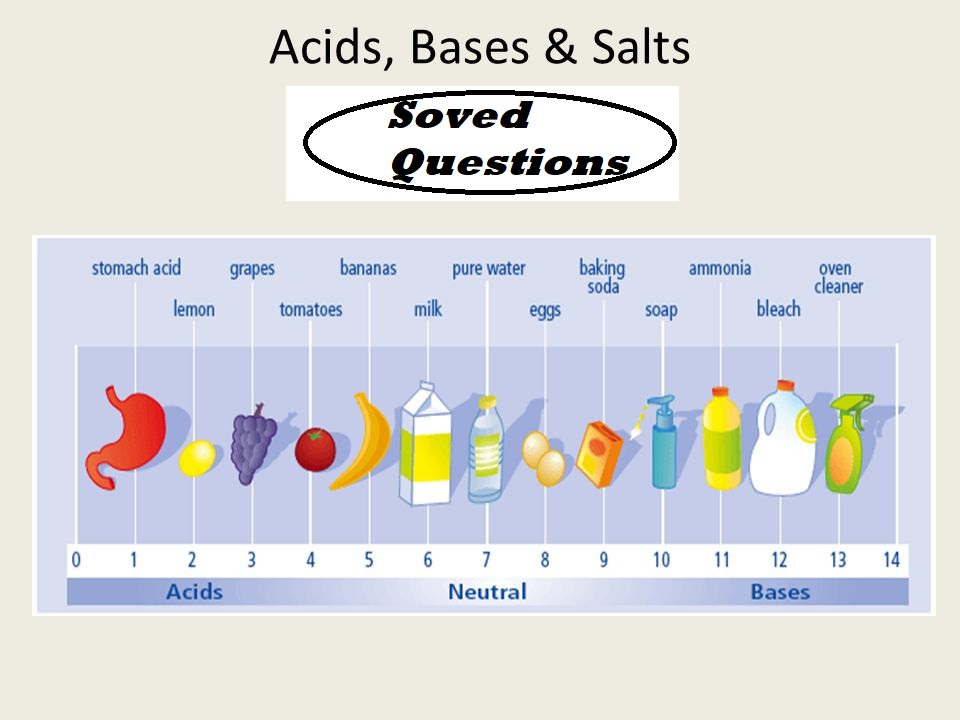
Acids Bases Salts Solved Questions All important questions are included in Acids Bases Salts Solved Questions. Que1. Define the terms: Acid, alkali and salt. Ans 1: An acid is a compound, which releases hydronium ions (H3O+) as the only positive ions in solution. An alkali is a compound, which releases hydroxyl ions (OH-) as the only negative ions in solution. A salt is one of the products of neutralization between an acid and a base; water being the only other product. OR A salt gives positive ions other than H+ ion and negative ions other than OH- ion in solution.…
Solid State Chemistry Numericals The post-Solid State Chemistry Numericals contain all important questions related to the topic Que 1. A solid has a cubic structure in which X atoms are located at the corners of the cube, Y atoms are at the cube centers and O atoms are at the edge centers. What is the formula of the compound? Ans 1: Atoms of X are present at all the eight corners of the cube. Therefore, each atom of X at the corner makes 1/8 contribution towards the unit cell. Number of atoms of X per unit cell = 8…
MCQs & Assertion Reason Haloalkanes and Haloarenes MCQs & Assertion Reason Haloalkanes and Haloarenes contain important questions of Haloalkanes and Haloarenes Q 1. Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution: (a) a < b < c (b) a < b < a (c) a < c < b (d) c < a < b Q…
Haloalkanes Methods of Preparation Haloalkanes Methods of Preparation Nature of C-X Bond: Since halogen atoms are more electronegative than carbon, the carbon halogen bond of alkyl halide is polarised; the carbon atom bears a partial positive charge whereas the halogen atom bears a partial negative charge. Haloalkanes Methods of Preparation: Alkyl halides are best prepared from alcohols, which are easily accessible. The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides, or thionyl chloride. From alcohols: a). By the action of concentrated halogen acids on alcohol in presence of anhydrous ZnCl2…
Chapter – 10 Haloalkanes and Haloarenes Haloalkanes and Haloarenes Syllabus: Introduction to Haloalkanes and Haloarenes Classification On the basis of the number of halogen atoms …
Solved worksheet Some Basic Concepts of Chemistry Solved worksheet Some Basic Concepts of Chemistry is all about subjective and objective knowledge. Q1. How many significant figures are there in (i) 3.070 and (ii) 0.0025? Ans 1. (i) 4 (ii) 2 Q 2. How many significant figures are present in the answer of the following calculation: .0125 + 0.8250 + 0.025 Ans 2. 0.86 Q 3. The density of vanadium is 5.96 g cm3. Express this in SI unit. Ans3. 5960 kg m3 Q 4. The body temperature of a normal healthy person is 370C. Calculate its value in 0F.…
Answers Subjective Assignment Chemical Reactions and Equations Ans 51. When lime water sticks on walls react with CO2 of air to forms a layer of CaCO3 which is white in colour. Ans 52. Both burning of Fuel and food are oxidation reaction, in burning of fuel CO2 and heat is released and in burning of Food, A.T.P. energy and CO2 are formed. Ans 53. Rusting is avoided in stainless steel because it is an alloy. Ans 54. In the cracker explosion, on burning fuel or material like charcoal, sulphur or potassium chloride reacts with oxygen in an oxidation reaction.…
Expressing Concentration Terms with Formula Concentration Can be Expressed in the Following Ways: (1) Mass percent (w/w or m/m): It is defined as the number of parts solute present in 100 parts by mass of solution. i.e. Mass % of a component = Mass of solute × 100 Mass of solution (2) Molarity (M): It is defined as the number of moles of solute dissolved per liter of solution. i.e. …