Solved Questions Electrochemistry Solved Questions Electrochemistry: Que 1. How much electricity in terms of Faraday is required to produce 100g of Ca from molten CaCl2? Ans 1. 5F Que 2. Name the solid substance produced, during the discharge of lead- storage battery. Ans 2. Lead sulphate Que 3. If 0.5 ampere current flows through a wire for 2 hours. Calculate the number of electrons through the wire. …
Author: Dr. Vikas Jasrotia
Primary Secondary Batteries and Corrosion Batteries: A battery is basically a galvanic cell in which the chemical energy of a redox reaction is converted to electrical energy. They are of mainly 2 types – primary batteries and secondary batteries. Primary batteries: Here the reaction occurs only once and after use over a period of time, they become dead and cannot be reused. E.g. Dry cell, mercury button cell, etc. (Primary cells cannot be recharged and reused). (i) Dry Cell (ii) Mercury Cell Primary Secondary Batteries and Corrosion Secondary cells: A secondary cell can be recharged and reused again and again. Here…
Solved MCQs Periodic Classification Solved MCQs Periodic Classification: Q1. Which of the following processes involves the absorption of energy? a) Cl + e- → Cl- b) O- + e‑ → O2- c) O + e- → O- d) S + e- → S- Ans 1. b) O- + e‑ → O2- As the electron is being added to the anion, whereas in all rest of three, the electron is being added to a –neutral atom which will not have as much repulsion as in the case of O-. Q2. Electronic Configuration of most electro negative element is a) 1s2…
NEET JEE Solved Questions Solid State NEET JEE Solved Questions Solid State: Q1. The three states of matter are solid, liquid, and gas. Which of the following statement is/are true about them [AIIMS 1991] (a) Gases and liquids have viscosity as a common property…
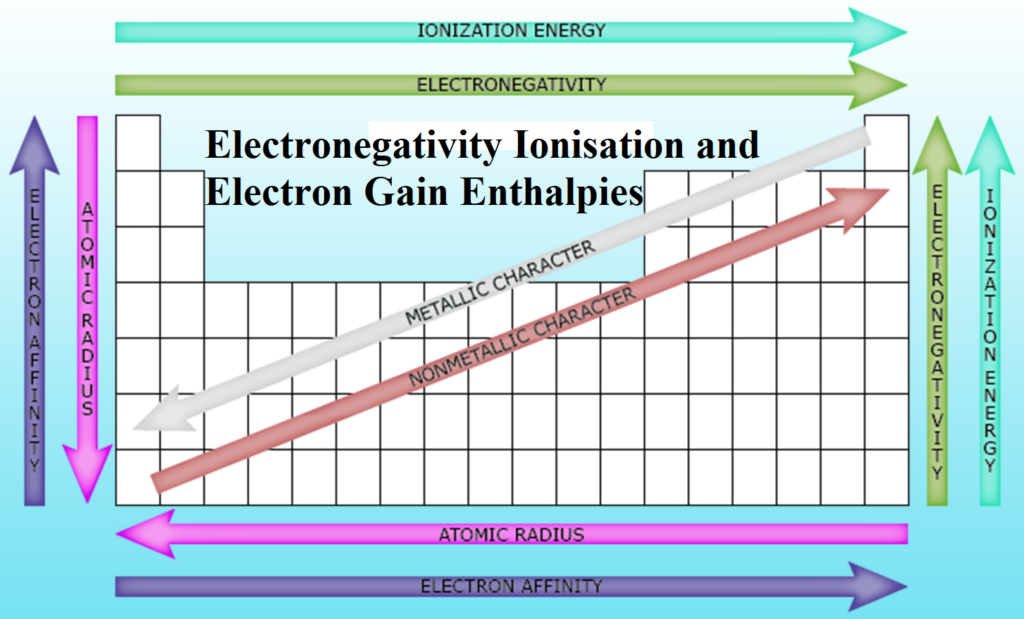
Electronegativity Ionisation and Electron Gain Enthalpies Electronegativity Ionisation and Electron Gain Enthalpies: Ionization Enthalpy (∆iH): Minimum amount of energy required to remove the most loosely bound valence electron from an isolated gaseous atom so as to convert it into gaseous cation. It may be represented as: X(g) + ∆iH → X+(g) + e– Its unit is kJ/mol or J/mol. The energy required to remove the first electron from the outermost shell of a neutral atom is called first ionization enthalpy (∆iH1) X(g) + ∆iH1 → X+(g) + e– Second Ionisation enthalpy (∆iH2) is the amount of energy required to remove…
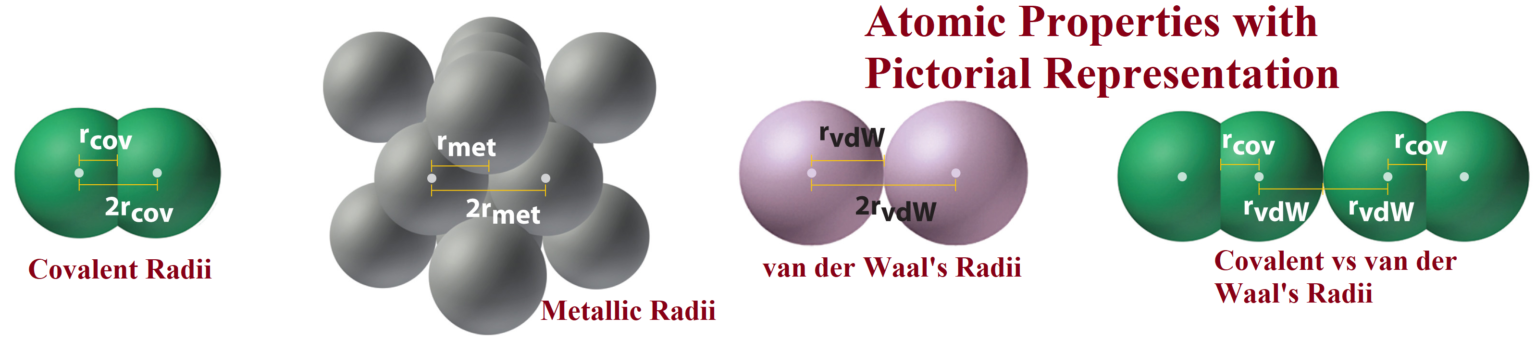
Atomic Properties with Pictorial Representation Atomic Properties with Pictorial Representation: Atomic Properties: The properties such as atomic radius, ionic radius, ionisation energy, electronegativity, electron affinity, and valence, called atomic properties. Atomic Radius: It is defined as the distance from the centre of the nucleus to the outermost shell containing electrons. Atomic radius is commonly expressed in picometre (pm) or angstrom (Å).it is measured by x-ray diffraction method or by spectroscopic methods. Covalent radius: One-half of the distance between the nuclei of two covalently bonded atoms of the same element in a molecule (used for non-metals). Van der Waals radius:…
Conductivity Molar conductivity and Electrolysis Resistance (R): The electrical resistance is a hindrance to the flow of electrons. Its unit is the ohm (Ω). The resistance of a conductor is directly proportional to the length of the conductor (Ɩ) and inversely proportional to the area of cross-section (A) of the conductor. R α Ɩ /A R = ρ Ɩ /A where ρ (rho) is a constant called resistivity or specific resistance. Its unit is ohm-meter (Ω m) or ohm-centimeter (Ω cm). Resistivity (ρ): It defined as the resistance offered by a conductor having unit length and unit area of…
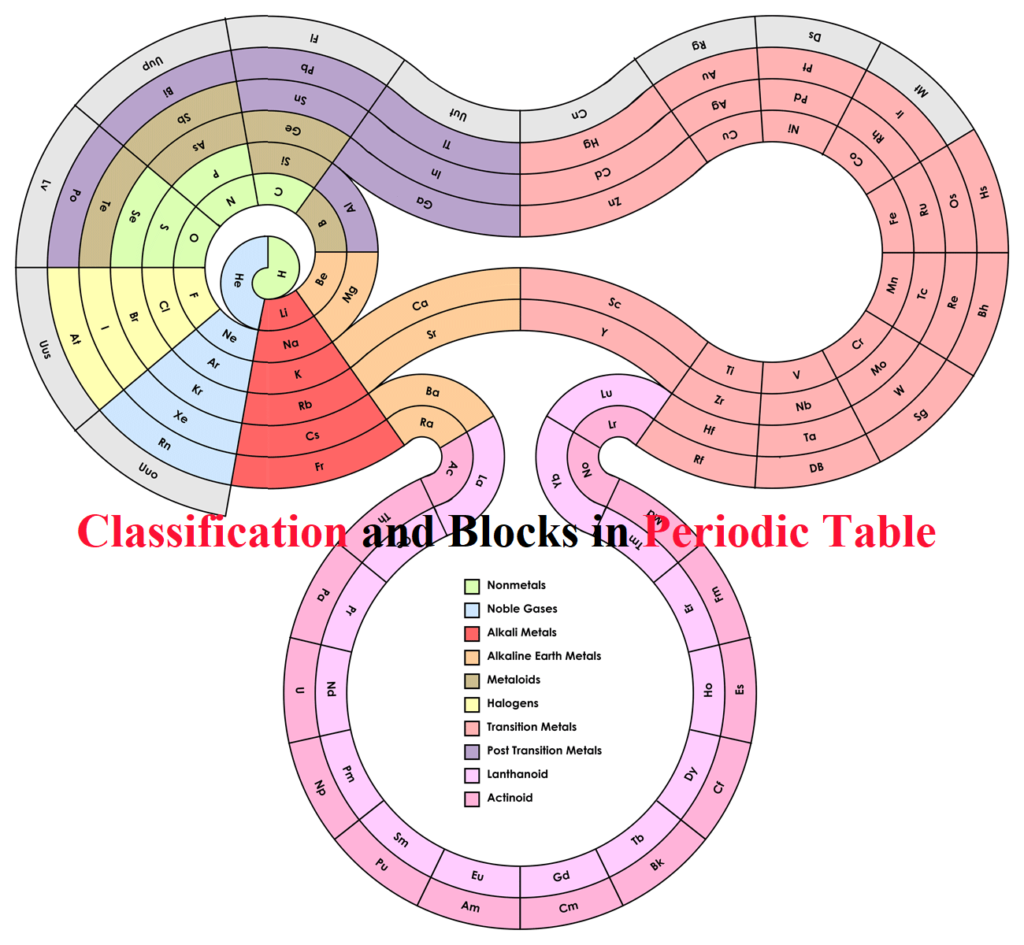
Classification and Blocks in Periodic Table Classification and Blocks in Periodic Table: Topics covered: Earlier classifications Dobereiner’s Triads Newland’s law of octaves Mendeleev’s Periodic Classification Modern Periodic Table or Moseley’s Periodic Law With the discovery of a large number of elements, it became difficult to study the elements individually, so the classification of elements was done to make the study easier. Earlier Classification: Dobereiner’s Triads: In triads, the atomic mass of the middle element is approximately the average of the other two elements. This is known as the Law of Triads. This classification was applicable to very few elements and…
Electrochemical Cells Nernst Equation and Gibbs Energy Syllabus:- Electrolytic cells & Galvanic cells The function of Salt Bridge Redox reaction, EMF of the cell, standard electrode potential Standard hydrogen electrode (SHE) Nernst equation and its application to Chemical cell Equilibrium Constant from Nernst Equation Electrochemical Cell and Gibbs Energy Electrochemistry: It is a branch of chemistry that deals with the relationship between chemical energy and electrical energy and their interconversions. Redox Reactions: Oxidation is the process that involves the loss of electrons & reduction is a process in which it involves the gain of electrons. The reactions which involve…
Solved Questions Structure of Atom Que 1. Neutrons can be found in all atomic nuclei except in one case. Which is this atomic nucleus and what does it consists of? Ans 1. Hydrogen atom. It consists of only one proton. Que 2. Calculate wave number of yellow radiations having a wavelength of 5800 A0. Ans 2. Wave number = 1/ wavelength Wavelength = 5800 A0= 5800 x 10-10 m Wave number = 1/5800 x 10-10 m = 1.72 x 106 m-1 Que 3. What are the values of n and l for 2p orbital? Ans 3. n=2 and l= 1…