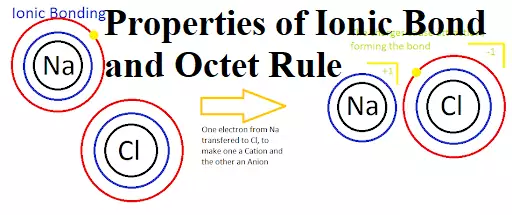
Properties of Ionic Bond and Octet Rule Properties of Ionic Bond and Octet Rule It is observed that the atoms of all the elements, except those for noble gases, tend to remain in a combined state with the atoms of the same or other elements. They do not exist as single atoms under ordinary conditions. Such atomic aggregates occur as molecules. The attractive force that binds the atoms together in a molecule is called a chemical bond. It is formed either by the transfer of electrons or by the sharing of electrons. The elements with one, two, three, four, five,…
Author: Dr. Vikas Jasrotia
Solved Questions Basic Concepts of Chemistry Solved Questions Basic Concepts of Chemistry Que 1. How many significant figures are there in (i) 3.070 and (ii) 0.0025? Ans 1. (i) 4 (ii) 2 Que 2. How many significant figures are present in the answer of the following calculation: .0125 + 0.8250 + 0.025 Ans 2. 0.86 Que 3. The density of vanadium is 5.96 g cm3. Express this in the SI unit. Ans 3. 5960 kg m3 Que 4. The body temperature of a normal healthy person is 370C. Calculate its value in 0F. Ans 4. We know 0F =…
The Colloidal State Preparation and Purification A colloid is an intermediate state between true solution and suspension. In a true solution, the size of the particles is < 1nm. The particles do not settle down under the influence of gravity or by any method and they cannot be filtered by a filter paper. Colloids are heterogeneous systems containing two phases – the dispersed phase and the dispersion medium. Dispersed Phase: The phase which is dispersed in the other (medium) is called the Dispersed Phase or internal phase, or discontinuous phase. Dispersion Medium: The phase or medium in which the dispersion…
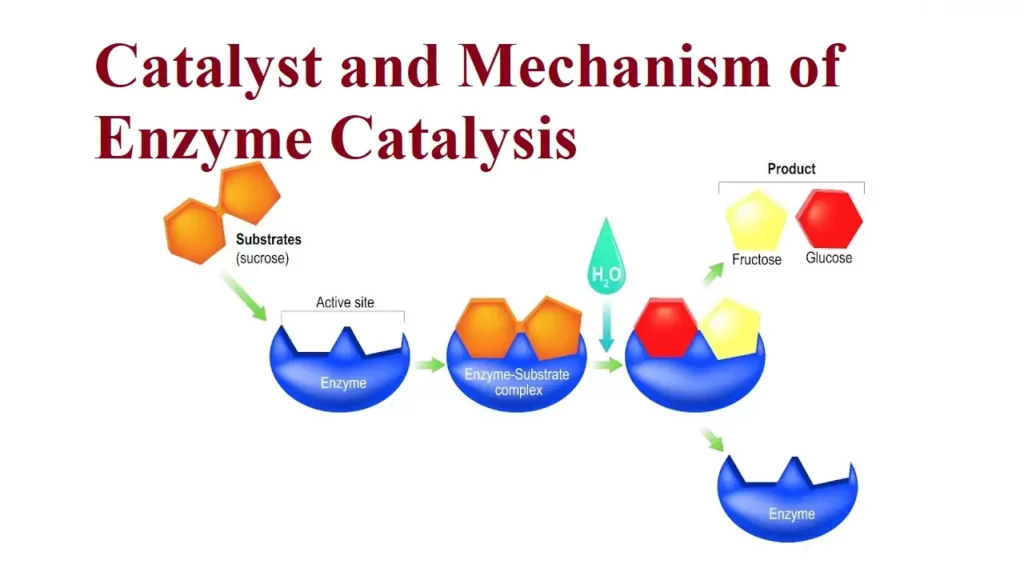
Catalyst and Mechanism of Enzyme Catalysis Catalysis: A catalyst is a substance that changes the rate of a chemical reaction without undergoing any permanent chemical change by itself. The process of changing the rate of a chemical reaction by a catalyst is known as Catalysis. Eg:- 2KClO3 + MnO2 → 2KCl+ 3O 473-633k Promoters and Poisons: Promoters are substances that enhance the activity of a catalyst while poisons decrease the…
Basic Concepts of Chemistry Notes Basic Concepts of Chemistry Notes Atoms and Molecules: Atom is the smallest particle of an element. Molecules are the smallest particle of a substance. A molecule has all the properties of that substance. Types of molecules Based on the type of atoms, there are two types of molecules – The homonuclear molecule and the Heteronuclear molecule. A molecule containing only one type of atom is called a Homonuclear molecule. e.g. H2, O2, N2, O3 (ozone), etc Heteronuclear molecules contain different types of atoms. E.g. CO2, H2O, C6H12O6, NH3, etc. Based on the no. of atoms…
Some Basic Concepts of Chemistry Notes Some Basic Concepts of Chemistry Notes Chemistry is the branch of science that deals with the properties, structure, and composition of matter. There are a large number of branches for Chemistry. Some of them are: Inorganic Chemistry Organic Chemistry Physical Chemistry Analytical Chemistry Polymer Chemistry Biochemistry Medicinal Chemistry Industrial Chemistry Hydrochemistry Electrochemistry Green Chemistry Some Basic Concepts of Chemistry Notes Matter: Matter is anything that occupies space, has a definite mass, and can be perceived by any of our sense organs. Based on the physical state we can divide matter into different categories. Solid-State …
Adsorption and Applications Surface Chemistry Surface Chemistry is the branch of chemistry that deals with the study of the phenomenon occurring at the surface than bulk. Adsorption: The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption. Adsorbate: The substance which is adsorbed is called adsorbate. Adsorbent: The substance whose surface on which adsorption takes place is called adsorbent. The commonly used adsorbents are charcoal, silica gel, alumina gel, clay, colloids, metals in a finely divided state, etc. Adsorption is a surface phenomenon. Some examples of adsorption are:…
MCQs Solved Chemical Kinetics MCQs Solved Chemical Kinetics: Que 1. The rate constant of a reaction is 0.005molL–1s–1. What is the order of this reaction? Ans 1. Zero-order reaction. Que 2. In a reaction, 2A → Products, the concentration of A decreases from 0.5mol L–1 to 0.4 mol L–1 in 10 minutes. Calculate the rate during this interval? Ans 2: Rate = – change in conc. of A/2 x time interval = – [0.4‐0.5]/2×10 = 0.005 mol L–1min–1 Que3. The rate constant for a first-order reaction is 60 s–1. How much time will it take to reduce the…
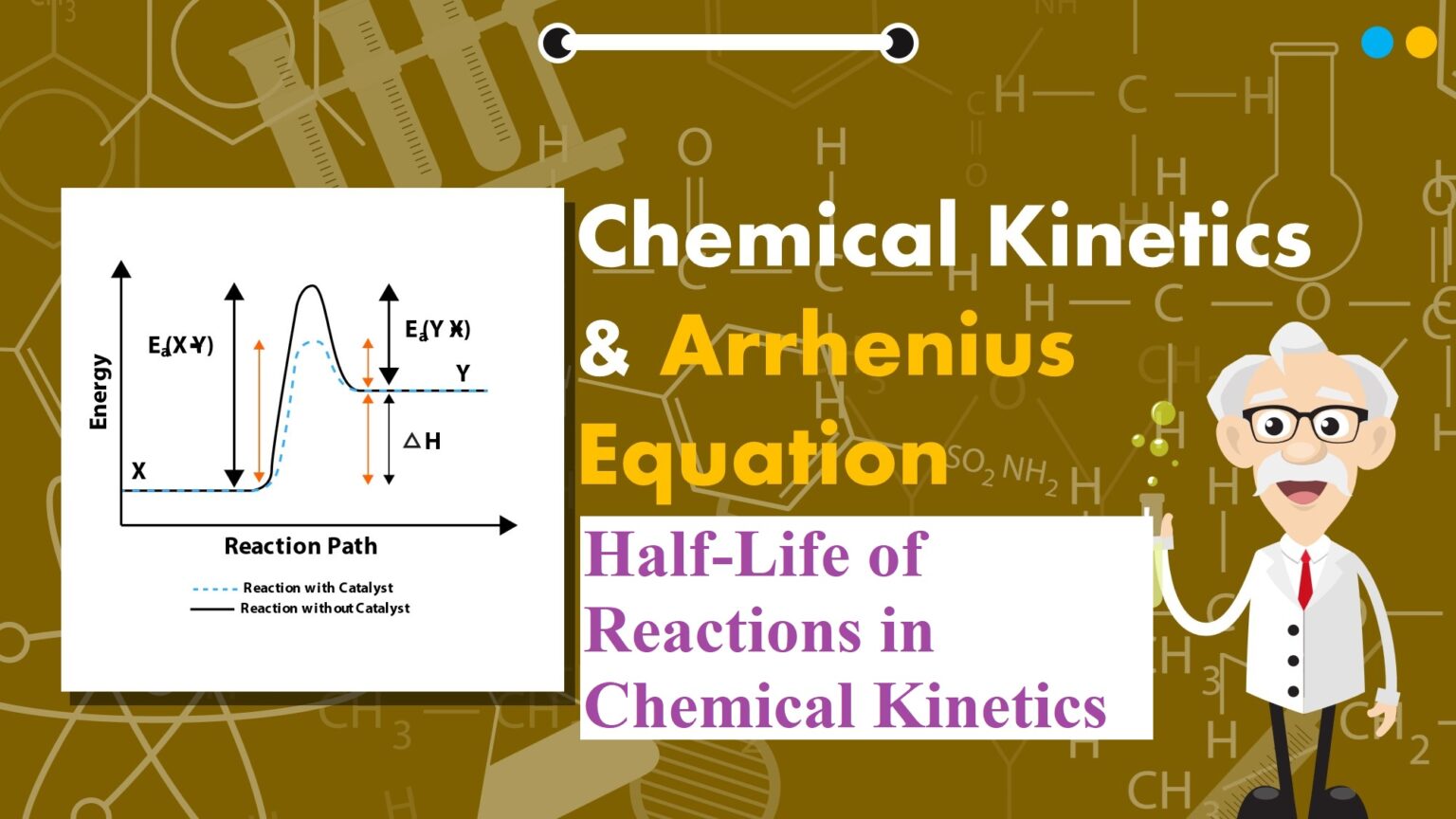
Half-Life of Reactions in Chemical Kinetics Half-Life of Reactions in Chemical Kinetics: Pseudo First Order Reaction: The reactions which are not truly of first order but become reactions of first-order under certain conditions are called pseudo first-order reactions. For example, Hydrolysis of ester (H+). CH3COOC2H5 + H2O → CH3COOH + C2H5OH Rate = k [CH3COOC2H5] [H2O] Since water is in excess and [H2O] is constant, so Rate = k[CH3COOC2H5], Where k = k’[H2O] Inversion of cane sugar (in acid) C12H22O11 + H2O → C6H12O6 + C6H12O6 Cane sugar …
Chemical Kinetics Rate and Order Notes Chemical Kinetics Rate and Order Notes: Chemical Kinetics: The branch of Chemistry that deals with the rate of chemical reaction, factors affecting the rate, and the mechanism of a reaction. Types of Reactions: On the basis of rates (i) Very fast reactions – e.g. precipitation of AgCl (ii) Very slow reactions – e.g. rusting of iron (iii) Reactions taking place at moderate speeds – e.g. hydrolysis of starch Rate of Chemical Reaction: It is the change in concentration of reactant or product with respect to time. OR It is the rate of decrease in…