Surface Chemistry Notes Class 12
Surface Chemistry is the branch of chemistry that deals with the study of the phenomenon occurring at the surface than bulk.
Adsorption: The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption.
Adsorbate: The substance which is adsorbed is called adsorbate.
Adsorbent: The substance whose surface on which adsorption takes place is called adsorbent.
The commonly used adsorbents are charcoal, silica gel, alumina gel, clay, colloids, metals in a finely divided state, etc.
Adsorption is a surface phenomenon. Some examples of adsorption are:
- Powdered charcoal absorbs gases like H2, O2, CO, Cl2, NH3, SO2
- Silica gel absorbs moisture
- Animal charcoal adsorbs colouring material from sugar solutions.
Desorption: The removal of the adsorbed substance from a surface is called desorption.

Sorption: If adsorption and absorption occur simultaneously, the process is called sorption.

Distinction Between Adsorption and absorption:


Mechanism of Adsorption:
The surface particles of the adsorbent are not in the same environment as the particles inside the bulk (inner part). Inside the adsorbent, all the forces are mutually balanced. But at the surface, there is always some unbalanced or residual forces. These forces of the adsorbent are responsible for adsorption.

Surface Chemistry Notes Class 12
Gibbs energy change during adsorption:
During adsorption, there is always a decrease in residual force i.e., there is a decrease in surface energy, which appears as heat. Therefore, adsorption is an exothermic process i.e. ΔH = – ve. Also, the movement of the particles is restricted in this process. Therefore ΔS = – ve According to Gibbs Helmholtz equation:
ΔG = ΔH – TΔS, or ΔG = (–ΔH ) – T(–ΔS) for adsorption to occur.
ΔG must be negative which is possible only when ΔH >TΔS.
Types of Adsorption:
(i) Physical adsorption: When the particles of adsorbate are held to the surface of the adsorbent by weak van der Waals forces.
Characteristics of physical adsorption: Lack of specificity, low enthalpy of adsorption, reversible in nature, no activation energy required, decrease with an increase in temperature.
(ii) Chemical adsorption: When the molecules of adsorbate are held to the surface of the adsorbent by strong chemical forces.
Characteristics of chemical adsorption: Highly specific in nature, high enthalpy of adsorption, Irreversible in nature, initially it increases with an increase in temperature as it needs activation energy, very slow.

Freundlich Adsorption Isotherm: The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm. (It is a plot/curve between the extent of adsorption (x/m) and pressure P at constant T).
The relationship can be expressed by the following equation:
x/m = k.P1/n (where n > 1)
where x is the mass of the gas adsorbed, m is the mass of the adsorbent, k and n are constants that depend on the nature of the adsorbent and the gas at a particular temperature.
The relationship can be expressed by the following equation:
x/m = k.P1/n (where n > 1)
where x is the mass of the gas adsorbed, m is the mass of the adsorbent, k and n are constants that depend on the nature of the adsorbent and the gas at a particular temperature.
The above relationship can be represented in the form of a graph as follows:
The plot of x/ m vs log P is a straight line with slope and intercept on Y-axis = log k.

Limitations of Freundlich Isotherm:
(i) It is unable to explain the deviation from linearity at high pressure.
(ii) It is applicable only for physical adsorption as it considers the multimolecular layer of adsorption.
(iii) It doesn’t consider the role of the surface area of adsorbent in the process of adsorption The Freundlich isotherm was modified by Langmuir in 1916.
Surface Chemistry Notes Class 12
Applications of Adsorption: The important applications of adsorption are:
(i) Production of high vacuum: For the complete evacuation of a vessel, activated charcoal is used.
(ii) Gas masks: The poisonous gases in coal mines can be removed by using gas masks containing activated charcoal.
(iii) Control of humidity: Silica and aluminium gels are used as adsorbents for removing moisture and controlling humidity.
(iv) Animal charcoal is used for the purification of cane sugar solution.
(v) Adsorption finds application in heterogeneous catalysis.
(vi) A mixture of noble gases can be separated by adsorption on coconut charcoal at different temperatures.
(vii) In curing diseases: A number of drugs are used to kill germs by getting adsorbed on them.
(viii) In the froth floatation process for the purification of sulphide ores in metallurgy.
(ix) Adsorption indicators like eosin, fluorescein, etc. are used in volumetric analysis.
(x) Chromatographic analysis for the separation of a mixture is based on adsorption.
Catalysis: A catalyst is a substance that changes the rate of a chemical reaction without undergoing any permanent chemical change by itself. The process of changing the rate of a chemical reaction by a catalyst is known as Catalysis.
Eg:- 2KClO3 + MnO2 → 2KCl+ 3O
473-633k
Promoters and Poisons: Promoters are substances that enhance the activity of a catalyst while poisons decrease the activity of a catalyst. For example, in Haber’s process for the manufacture of ammonia, molybdenum (Mo) acts as a promoter for the catalyst iron.
N2 + 3H2 → 2NH3
Poisions are those substances that decrease the activity of catalyst are called catalytic poisons or inhibitors. Eg- arsenic acts as a catalytic poison in the manufacture of sulphuric acid by contanct process.
Surface Chemistry Notes Class 12
Types of Catalysis:
Positive and Negative Catalyst
A catalyst that increases the rate of a chemical reaction is called a Positive catalyst and that decreases the rate of a chemical reaction is called a negative catalyst (inhibitors).
E.g. In Haber’s process for the manufacture of ammonia, Fe acts as a positive catalyst and Mo as a promotor
N2 +3H2 Fe/Mo 2NH3
→
For decreasing the rate of dissociation of H2O2, Phosphoric acid is used as a negative catalyst.
Homogenous and Heterogeneous Catalysis:
Homogeneous Catalysis
A catalytic process in which the reactants and the catalyst are in the same phase (i.e., liquid or gas), is said to be homogeneous catalysis.
e.g.: In the lead chamber process for the manufacture of Sulphuric acid, oxidation of sulphur dioxide into sulphur trioxide is done in the presence of Nitric Oxide as a catalyst.
2SO2+ O2 → SO3
Heterogeneous Catalysis
The catalytic process in which the reactants and the catalyst are in different phases is known as heterogeneous catalysis. Example of heterogeneous catalysis:
In the contact process for the manufacture of H2SO4, Oxidation of sulphur dioxide into sulphur trioxide is done in presence of V2O5.
2SO2(g) + O2(g) → 2SO3(g)
Adsorption Theory of Heterogeneous Catalysis:
This theory explains the mechanism of heterogeneous catalysis. According to this theory, the catalytic activity takes place on the surface of the catalyst. The mechanism involves five steps:
- Diffusion of reactants to the surface of the catalyst.
- Adsorption of reactant molecules on the surface of the catalyst.
- The occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate.
- Desorption of reaction products from the catalyst surface.
- Diffusion of reaction products away from the catalyst’s surface.

Surface Chemistry Notes Class 12
Features of Solid Catalysts:
(i) Activity: The activity of a catalyst depends upon the strength of chemisorption to a large extent. The reactants must get adsorbed reasonably strongly on to the catalyst to become active. Eg‐ 2H2O + O2 → 2H2O
(ii) Selectivity: The selectivity of a catalyst is its ability to direct a reaction to yield a particular product.

Shape-Selective Catalysis by Zeolites: The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis. Zeolites are good shape-selective catalysts because of their honeycomb-like structures. They are microporous aluminosilicates with three-dimensional networks of silicates in which some silicon atoms are replaced by aluminium atoms. They contain Al–O–Si framework. The reactions taking place in zeolites depend upon the size and shape of reactant and product molecules as well as upon the pores and cavities of the zeolites. They are found in nature as well as prepared artificially.
Zeolites are used as catalysts in petrochemical industries for the cracking of hydrocarbons and isomerisation. An important zeolite catalyst used in the petroleum industry is ZSM-5. It converts alcohols directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
Enzyme Catalysis:
Enzymes are complex nitrogenous organic compounds that are produced by living plants and animals. They are actually protein molecules of high molecular mass. They are very effective catalysts and catalyse numerous reactions taking place in plants and animals. So enzymes are also called Biochemical Catalysts and the phenomenon is known as Biochemical Catalysis.
For Example:
(i) Inversion of cane sugar: The enzyme invertase converts cane sugar into glucose and fructose.
C12H22O11(aq) + H2O(l) → C6H12O6(aq) + C6H12O6(aq)
(ii) Conversion of glucose into ethyl alcohol: The enzyme zymase converts glucose into ethyl alcohol and carbon dioxide.
C6H12O6(aq) → 2C2H5OH(aq) + 2CO2(g)
Characteristics of Enzyme Catalysis:
(i) Enzymes are specific in action.
(ii) The optimum temperature for enzymatic action is 298 to 310 K.
(iii) They show maximum activity near pH 5 – 7.
(iv) The enzymatic activity is increased in the presence of certain substances, known as co-enzymes.
(v) Enzyme activity is highly efficient. i.e., one molecule of an enzyme may transform one million molecules of the reactant per minute.
Mechanism of Enzyme Catalysis:
This theory is known as lock and key theory. Thus, the enzyme-catalysed reactions may be considered to proceed in two steps.
Step 1: The enzyme combines with the substrate to form an activated complex.
- E+S→ES*
Step 2: Decomposition of the activated complex to form the product.
The schematic representation of the mechanism of enzyme catalysis is as follows:

Surface Chemistry Notes Class 12
A colloid is an intermediate state between true solution and suspension. In a true solution, the size of the particles is < 1nm. The particles do not settle down under the influence of gravity or by any method and they cannot be filtered by a filter paper.

Colloids are heterogeneous systems containing two phases – the dispersed phase and the dispersion medium.
Dispersed Phase: The phase which is dispersed in the other (medium) is called the Dispersed Phase or internal phase, or discontinuous phase.
Dispersion Medium: The phase or medium in which the dispersion is made is called dispersion medium or external phase or continuous phase.
Classification colloids
(i) Based on the physical state of the dispersed phase and the dispersion medium:
Depending upon the physical state of the dispersed phase and the dispersion medium, there are eight types of colloidal systems.
| Dispersed Phase | Dispersion medium | Type of colloid | Examples |
| Solid | Solid | Solid sol | Some coloured glasses, gems stones |
| Solid | Liquid | Sol | Paints, muddy water, cell fluids |
| Solid | Gas | Aerosol | Smoke, dust |
| Liquid | Solid | Gel | Cheese, butter, jellies |
| Liquid | Liquid | Emulsion | Milk, hair cream |
| Liquid | Gas | Aerosol | Fog, mist, cloud, insecticide sprays |
| Gas | Solid | Solid foam | Pumice stone, foam rubber |
| Gas | Liquid | Foam | Froth, whipped cream, soap lather |
(ii) Based on the nature of the dispersion medium:
| Dispersion medium | Name of colloid (Sol) |
| Air | Aerosol |
| Water | Hydrosol |
| Alcohol | Alcosol |
| Benzene | Benzosol |
Based on the attraction between the dispersed phase and the dispersion medium:
On the basis of the nature of the interaction of the dispersed phase and the dispersion medium colloids are of two types: lyophilic (solvent loving) and lyophobic (solvent hating). If the force of attraction between the dispersed phase and dispersion medium is strong, it is called lyophilic sol e.g. gum, gelatin, starch, rubber etc in a suitable dispersion medium.
If the force of attraction between the dispersed phase and dispersion medium is weak, it is called a lyophobic sol. e.g. Arsenic sulphide (As2S3) sol, Sulpher sol and metal sols like gold sol, silver sol etc.
Difference Between Lyophilic and Lyophobic Sols:
| Properties | Lyophilic sol | Lyophobic sol |
| Force of attraction | Strong | Weak |
| Preparation | Can be easily prepared by mixing the dispersed phase with the dispersion medium | Some special methods are used for the |
| Reversibility | Reversible (i.e. they can be easily separated and remixed. | Irreversible |
| Stability | Self-Stabilized | Less stable and requires some stabilizing agent |
| Coagulation | a large amount of electrolyte is required for coagulation | Only a small amount of electrolyte is required. |
Based on Nature of Particles:
On this basis, the colloids are classified into three types namely Multimolecular colloids, Macromolecular colloids and Associated colloids.
Multimolecular colloids. They are formed by the aggregation of a large number of atoms or molecules which generally have a diameter less than 1nm, e.g., sols of gold, sulphur etc. Their atoms or molecules are held together by weak van der Waals forces and their molecular masses are not high.
Macromolecular colloids. They are molecules of large size, e.g., polymers like rubber, nylon, polythene, starch, cellulose, proteins, enzymes, etc. These substances when dissolved in a suitable liquid, directly from the colloidal solution. They have high molecular masses and have lyophobic character.
Associated colloids: The substances which when dissolved in a medium at low concentration behave as normal strong electrolytes but at higher concentration exhibit colloidal state properties due to the formation of aggregated particles are called associated colloids. The aggregated particles thus formed are called micelles.
Micelle formation takes place above a particular temperature called Kraft temperature (Tk) and above a particular concentration called Critical Micelle Concentration (CMC). These molecules contain both lyophilic and lyophobic groups.
Mechanism of micelle formation:
An example of the micelle is a soap solution. Soap is sodium or potassium salt of higher fatty acid and may be represented as RCOO–Na+. When dissolved in water, it dissociates into RCOO– and Na+ ions. The RCOO– ions consist of two parts – a long hydrocarbon chain R (also called non-polar ‘tail’) which is hydrophobic (water-repelling), and a polar group COO– (also called polar-ionic ‘head’), which is hydrophilic (water-loving).
The RCOO– ions are present on the surface with their COO– groups in water and the hydrocarbon chains (R) at the surface. But at critical micelle concentration, the anions are pulled into the bulk of the solution and aggregate to form a spherical shape. Thus, a micelle is formed.
Cleansing Action of Soaps: The cleansing action of soap is due to micelle formation. The soap molecules form micelle around the oil droplet in such a way that the hydrophobic part is in the oil droplet and the hydrophilic part projects out. Since the polar groups (hydrophilic end) can interact with water, the oil droplets are pulled in water and removed from the dirty surface. Thus, soap helps in emulsification and washing away of oils and fats.

Surface Chemistry Notes Class 12
Preparation of colloids:
Some of the methods used for the preparation of colloids are:
Preparation of lyophilic sols: Prepared by dissolving these substances (e.g. starch, gelatine, glue etc.) in water either in cold or warm. Solutions of colloidal electrolytes (e.g. soaps, dyes) are also prepared in a similar manner.
Preparation of lyophobic sols: To obtain a substance in the colloidal form either the substance in bulk is broken down into particles of colloidal dimension (1nm to 1000nm) or the size of molecular particles is increased to colloidal dimensions. In some cases, another substance is added to increase the stability of sol. Such substances are known as a stabilizer. Thus, there are two ways by which lyophobic sols can be prepared.
(i) Chemical methods: Colloidal solutions can be prepared by chemical reactions like oxidation, reduction, double decomposition, hydrolysis etc.
- Oxidation: Sulphur sol can be prepared by passing H2S gas through an aqueous solution of sulphur dioxide. SO2 + 2H2S → 3S(sol) + 2H2
- Reduction: Sols of metals like silver, gold and platinum are obtained by the reduction of their salts with reducing agents like formaldehyde, stannous chloride etc.
2AuCl3 + 3HCHO + 3H2O → 2Au(sol) + 3HCOOH + 6HCl
- Hydrolysis: Ferric hydroxide sol is obtained when the concentrated solution of ferric chloride is added drop-wise to hot water.
FeCl3 + 3H2O → Fe(OH)3 (sol) + 3HCl
- Double Decomposition: A colloidal solution of arsenic sulphide is formed by passing H2S through a dilute solution of arsenious oxide in water.
As2O3 + 3H2S → As2S3(sol) + 3H2O
(ii) Electrical disintegration (Bredig’s arc method): This method is used for the preparation of metal sols like Ag, Au, Pt etc. The metal whose sol is to be prepared is taken in the form of two rods and it is kept in a suitable dispersion medium containing a small amount of electrolyte. The whole arrangement is kept in an ice bath. When high voltage is passed through the metal, the intense heat produced vapourises the metal, which then condensed to form particles of colloidal dimension.

(iii) Peptisation: It is the process of converting a freshly precipitated substance into colloidal particles by shaking with a suitable electrolyte, e.g., Fe(OH)3 ppt with FeCl3 solution, AgI ppt with AgNO3 or KI solution and Al(OH)3 ppt . with an insufficient quantity of very dil. HCl solution. During peptization, the precipitate adsorbs one of the ions of the electrolyte on its surface resulting in the development of positive or negative charge on the precipitate, which ultimately breaks into particles of colloidal dimensions.
Surface Chemistry Notes Class 12
Purification of Colloids:
The colloidal solution prepared contains an excess amount of electrolyte and some other soluble impurities. Even though a small amount of electrolyte is required for the stability of colloid, a large amount may cause precipitation. The process of reducing the concentration of electrolyte and other impurities is known as the purification of colloids. Some methods used for purification are:
(i) Dialysis: The process of removing the impurities from a sol by means of diffusion through a semi-permeable membrane is called dialysis.

(ii) Electrodialysis: When the dialysis process is accelerated by the application of a potential difference across the membrane, so ions migrate faster than the colloids.

(iii) Ultra-filtration: Purification of colloidal solution using special filter paper called ultrafilters (filter paper which is impregnated with gelatin or collodion followed by hardening in formaldehyde). Collodion is a 4% nitrocellulose soln in alcohol and ether.
Properties of Colloids
(i) Heterogeneous nature: Colloids are heterogeneous in nature and consists of two phases, the dispersed phase and the dispersion medium.
(ii) Visibility: The particles are too small to be seen with the naked eye but become visible when viewed through an ultra-microscope due to the scattering of light by them.
(iii) Surface tension and Viscosity: The surface tension and viscosity of lyophobic sols are not very different from that of the dispersed medium. But, lyophilic sols show higher viscosity and lower surface tension in comparison to the dispersion medium.
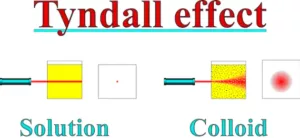
(iv) Tyndall Effect: When a light beam is passed through a colloidal solution, we can see the path of the light beam. This phenomenon is known as the Tyndall effect. It is due to the scattering of a light beam by colloidal particles. The visible path is called the Tyndall cone.
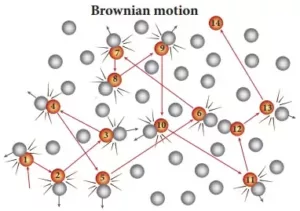
(v) Brownian movement: Zig-zag movement of colloidal particles due to the collision between particles of dispersed phase & dispersion medium, responsible for the stability of colloids.
Stability of Colloidal Sols: All the dispersed particles in a colloid carry the same charge (either positive or negative) while the dispersion medium has an equal and opposite charge. The particles, therefore, repel one another and do not come close together to form large non-colloidal particles.
The charge on colloidal particles is due to the preferential adsorption of ions, e.g., Fe(OH)3 adsorbs Fe3+ ions from FeCl3 solution and forms positively charged sol. Similarly, AgCl particles can adsorb Cl− ions from chloride solutions or Ag+ ions from solutions having silver ions. The sol will be negatively charged in the first case and positively charged in the second case.
- Negatively charged colloidal sols: Metallic particles like Cu, Au, Pt, Ag etc., starch, clay, silicic acid, metal sulphides like As2S3, CdS, Sb2S3, acidic dyes like congo red, eosin etc.
- Positively charged colloidal sols: Metal hydroxides like Fe(OH)3, Al(OH)3, Cr(OH)3, Ca(OH)2, oxides like TiO2, haemoglobin and basic dyes like methylene blue.
The charge on the sol particles is mainly due to the preferential adsorption of ions from the solution. When 2 or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle takes place. e.g. When AgNO3 is added to KI, AgI is precipitated, which adsorbs iodide ions from the dispersion medium and thus get a negative charge.
But when KI is added to AgNO3, the precipitated AgI adsorbs Ag+ ions from the solution and thus get a positive charge.
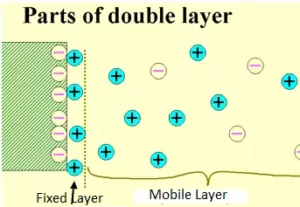
Due to the positive or negative charge in the sol particles, they attract the counterions (opposite ions) from the medium. Thus, a double layer of opposite charges is formed. This is known as a Helmholtz electrical double layer. The layer in which the ions are directly adsorbed to the sol particles is termed as a fixed layer. The second layer is mobile and is termed a diffused layer.
Due to the opposite charges on the fixed and diffused layers, there arises a potential difference between these layers. This potential difference between the fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or zeta potential.
Electrophoresis:
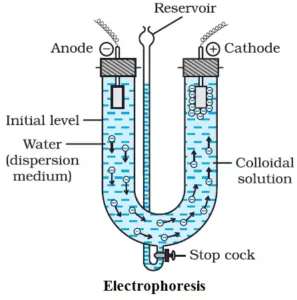
Since colloidal particles carry a charge, they move under the influence of an electric field. This movement of colloidal particles is called electrophoresis. The positively charged sol particles move towards cathode (cataphoresis) and the negatively charged particles move towards the anode (anaphoresis).
Coagulation of colloidal solutions: Process of settling of colloidal particles into precipitate by addition of electrolytes. This can be done in different ways:
- By electrophoresis
- By mixing two oppositely charged sols
- By continuous dialysis
- By boiling
- By the addition of electrolyte
When an electrolyte is added to the sol, the ions carrying the opposite charge to that of the sol neutralize the charge and causes precipitation. The ion of the electrolyte which causes the precipitation is called the coagulating ion or the flocculating ion. A negatively charged ion causes the precipitation of positively charged sol and vice versa
Generally, the greater the valency of the coagulating ion, the greater will be the coagulating power. This is known as Hardy – Schulze rule.
For negatively charged sol, the coagulating power of electrolytes are:
AlCl3 > BaCl2 > NaCl or Al3+> Ba2+> Na+
For positively charged, then the coagulating power of electrolytes follow the following order: K4[Fe(CN)6] > PO43– > SO42– > Cl–.
Coagulating value: The minimum concentration of an electrolyte in millimoles per litre required for the coagulation of a sol within 2 hours is called coagulating value. The smaller the coagulating value, the higher will be the coagulation power.
Protection of colloids: Lyophilic sols are self stabilized, while lyophobic sols require some stabilizing agents. For this purpose, some lyophilic sols are added to lyophobic sols. These lyophilic sols are called protective colloids. When a lyophilic sol is added to a lyophobic sol, the lyophilic particles form a layer around lyophobic particles and thus protect them from electrolytes.
| Difference between Flocculation and Coagulation: When a small amount of the electrolyte is added, i.e., when the concentration of the electrolyte added is low, the process is called flocculation. It can be reversed on shaking. However, at higher concentration, coagulation takes place and the process cannot be reversed simply by shaking. |
Surface Chemistry Notes Class 12
Emulsions: It is a colloidal dispersion in which both the dispersed phase and the dispersion medium are liquids (which are otherwise immiscible). Substances like soaps that help in making the emulsions stable are called emulsifiers or emulsifying agents.
Types of Emulsions: Emulsions are of two types:
1. Oil in water (o/w): Emulsions in which oil is the dispersed phase and water is the dispersion medium. For example, milk and vanishing cream.
2. Water in oil (w/o): Emulsions in which water is the dispersed phase and oil is the dispersion medium. For example, cod liver oil, butter and cream.
Dye test to distinguish between Emulsions:
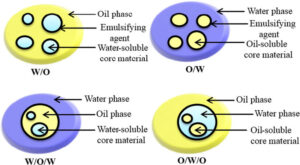
Demulsification: The process of breaking an emulsion to yield constituent.
Surface Chemistry Notes Class 12
Properties and Applications of Colloids
Applications of Colloids:
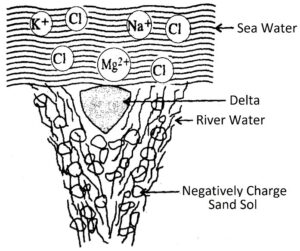
(1) Formation of Delta: Deltas are formed at the river mouth. This is because the river water is a negatively charged colloid of sand particles. When this water enters into the sea, the positive ions present in seawater coagulate the colloidal solution of sand and so the particles settle down. This will result in the formation of the delta.
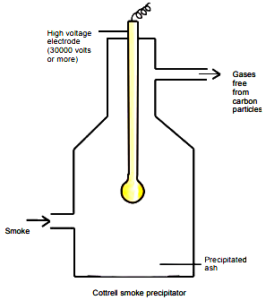
(2) Electrical precipitation of smoke (Cottrell precipitation): Smoke is a colloidal solution of carbon, arsenic compounds, dust particles etc. in the air. The smoke before coming out of the chimney is passed through a chamber (Cottrell precipitator) containing plates having a charge opposite to that of smoke particles. Thus, neutralization of charges occurs and the particles settle down and pure air flows out of the chimney.
(3) Purification of drinking water: The water obtained from natural sources often contains suspended impurities. In order to coagulate these impurities, alum is added to water. The positive ions present in alum neutralize the suspended impurities and hence get purified.
(4) Medicines: Most of the medicines are colloidal in nature. This is because they have a large surface area and are therefore easily assimilated. For example, Argyrol is a silver sol used as an eye lotion. Colloidal antimony is used in curing kala-azar (Visceral disease, the most devastating and fatal form of leishmaniasis, is classically known as kala-azar or the Indian name for “black fever/disease). Colloidal gold is used for intramuscular injection.
(5) Tanning: Animal hides are colloidal in nature. When a hide, which has positively charged particles, is soaked in tannin (which contains negatively charged colloidal particles) mutual coagulation takes place. This results in the hardening of leather. This process is termed as tanning.
(6) Photographic plates and films: Photographic plates or films are prepared by coating an emulsion of the light-sensitive silver bromide in gelatin over glass plates or celluloid films.
(7) Rubber industry: Rubber latex is a colloidal solution of rubber particles that are negatively charged. Rubber is obtained by coagulation of the latex.
(8) Food articles: Milk, butter, halwa, ice creams, fruit juices, etc., are all colloids in nature.
(9) Blood: Blood is a colloidal solution of an albuminoid substance. When alum and ferric chloride (FeCl3) solution are added to blood, then coagulation of particles takes place which results in the clotting of blood.
Surface Chemistry Notes Class 12