Assignment – 1 Chemical Reactions and Equations
This subjective Assignment is basically focused to check the reasoning and logical aptitude of the students. Assignment – 1 Chemical Reactions and Equations also focused to cover wide portion of chapter.
- Identify the type of chemical reaction
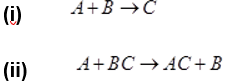
- Why does not silver evolve hydrogen on reacting with dil H2SO4?
- Why do diamond and graphite, the two allotropic forms of carbon evolve different amounts of heat on combustion?
- What is the role of oxidizing agent is a reaction?
- What happens chemically when quick lime is added to water?
- Why a combustion reaction an oxidation reaction?
- Why are food particle preferably packed in aluminium foil?
- What happens to lime water when Co2gas is bubbled through it in excess?
- Why is a Combustionreaction an oxidation reaction?
- Identify the type of chemical reaction
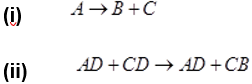
- Why cannot a chemical change be normally reversed?
- Identify the substance oxidized and reduced in the reaction.
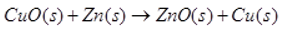
- A student took 23 g of a substance X in a glass beaker & poured water over it slowly. He observed bubbles along with hissing noise. The beaker becomes quite hot. Identify X. What type of reaction is it?
- A substance X used for coating iron articles is added to a blue solution of a reddish brown metal Y,the color of the solution gets discharged Identify X and Y & also the type of reaction.
- A student burnt a metal A found in the form of a ribbon. The ribbon burnt with a dazzling Flame & a white powder B is formed which is basic in nature. Identify A & B. Write the Balanced chemical equation.
Answers Assignment 1 – Chemical Reactions and Equations